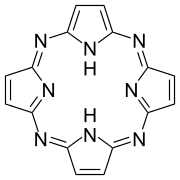
Porphyrazine
Porphyrazines, or tetraazaporphyrins, are tetrapyrrole macrocycles similar to porphyrins and phthalocyanines. Pioneered by Sir R. Patrick Linstead as an extension of his work on phthalocyanines,[1] porphyrazines differ from porphyrins in that they contain -meso nitrogen atoms, rather than carbon atoms, and differ from phthalocyanines in that their β-pyrrole positions are open for substitution. These differences confer physical properties that are distinct from both porphyrins and phthalocyanines.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Porphyrazine | |
| Other names
5,10,15,20-Tetraazaporphine; Tetraazaporphine; Tetraazaporphyrin; Tetrazaporphin | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H10N8 | |
| Molar mass | 314.312 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
Porphyrazines are prepared by magnesium templated cyclization of maleonitriles.[3] Cross-cyclization with phthalonitrile or diiminoisoindole derivatives is possible introducing a flexibile synthetic route that has led to the synthesis of porphyrazines with peripheral heterocyclic rings,[4] heteroatom substituents (S, O, N),[5] peripherally bound metal atoms,[6] and mixed -benzo porphyrazine systems.[7]
Optical properties
Porphyrazines are most well known for their intense electronic absorption throughout the UV, visible, and NIR spectral regions. Electronic absorption spectra for porphyrazines are similar to those of phthalocyanines,[8] with an intense Soret band (λ ≈ 300 - 400 nm) and Q-band (λ > 600 nm).[7][9]
Porphyrazines exhibit fluorescence from the first excited singlet state (S1 → S0)[10] at visible and NIR wavelengths which is typical of tetrapyrrole macrocycles. Dual-emission from organic fluorophores is not common but, as observed in phthalocyanines,[11][12] violet emission from an upper excited state (S2 → S0) is observed in porphyrazines.[13][14][15]
References
- Cook, A. H.; Linstead, R. P., "Phthalocyanines. XI. The preparation of octaphenylporphyrazines from diphenylmaleinitrile." J. Chem. Soc. 1937, 929-933.DOI: 10.1039/JR9370000929
- Ghosh, A.; Fitzgerald, J.; Gassman, P.G.; Almof, J. Inorg. Chem., 1994, 33, 6057-6060. DOI: 10.1021/ic00104a014
- Kobayashi, N. Meso-Azaporphyrins and Their Analogues. In The Porphyrin Handbook, Kadish, K.M.; Smith, K.M.; Guilard, R., Eds.; Academic Press; 1999, Vol. 2, pp. 317-321.
- Angeloni, S.; Ercolani, C., New classes of porphyrazine macrocycles with annulated heterocyclic rings. Journal of Porphyrins and Phthalocyanines, 2000, 4, 474–483.
- Michel S.L.J.; Hoffman B.M. Baum S.M.; Barrett A.G.M.; "Peripherally functionalized porphyrazines: Novel metallomacrocycles with broad, untapped potential;" Progress in Inorganic Chemistry, 50: 473-590, 2001.
- For example: Zhao, M; Zhong, C.; Stern, C.; Barrett, A.G.M.; Hoffman, B.M., Synthesis and Properties of Dimetallic M1[Pz]-M2[Schiff Base] Complexes. Inorg. Chem., 2004, 43, 3377-3385.
- Miwa, H., Ishii, K., Kobayashi, N., Electronic Structures of Zinc and Palladium Tetraazaporphyrin Derivatives Controlled by Fused Benzo Rings. Chemistry - A European Journal, 2004,10, 4422–4435.
- Kobayashi, N. and H. Konami (1996) Molecular orbitals and electronic spectra of phthalocyanine analogues. In Phthalocyanines: Properties and Applications, Vol. 4 (Edited by C. C. Leznoff and A. B. P. Lever), pp. 343–404. VCH Publishers, Inc., New York.
- Linstead, R. P.; Whalley, M., Conjugated Macrocycles. Part XXI Tetrazaporphin and its Metallic Derivatives. J. Chem. Soc. 1952, 4839-4844.
- Shushkevich, I.K.; Pershukevich, P.P.; Stupak, A.P.; Solov'ev, K.N.; Journal of Applied Spectroscopy, 2005, 72, 767-770.
- Chahraoui, D., Valat, P., and Kossanyi, J., Fluorescence of Phthalocyanines: Emission from an Upper Excited State. Res. Chem. Intermed., 1992, 17, 219-232.
- Kaneko, Y., Nishimura, Y., Takane, N., Arai, T., Sakuragi, H., Kobayashi, N., Matsunaga, D., Pac, C., and Tokumaru, K., Violet emission observed from phthalocyanines. J. Photochem. Photobiol. A, 1997, 106, 177-183.
- Lee, S., White, A.J.P., Williams, D.J., Barrett, A.G.M., and Hoffman, B.M. Synthesis of Near-IR Absorbing/Emitting Porphyrazine Derivatives with Tunable Solubility. J. Org. Chem., 2001, 66, 461-465.
- Lee, S.; Stackow, R.; Foote, C.S.; Barrett, A.G.M.; Hoffman, B.M.; Tuning the Singlet Oxygen Quantum Yield of Near-IR-absorbing Porphyrazines. Photochem. Photobio., 2003, 77, 18-21.
- Trivedi, E.R., Vesper, B.J., Weitman, H., Ehrenberg, B., Barrett, A.G.M., Radosevich, J.A., and Hoffman, B.M., Chiral bis-Acetal Porphyrazines as Near-infrared Optical Agents for Detection and Treatment of Cancer. Photochem. Photobio., 2010, 86, 410-417.