Potassium amyl xanthate
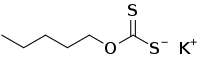
Potassium amyl xanthate (/pəˈtæsiəm ˌæmɪl ˈzænθeɪt/) is an organosulfur compound with the chemical formula CH3(CH2)4OCS2K. It is a pale yellow powder with a pungent odor that is soluble in water. It is widely used in the mining industry for the separation of ores using the flotation process.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Potassium O-pentyl carbonodithioate | |
| Other names
potassium pentylxanthogenate potassium-O-pentyl dithiocarbonate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| C6H11KOS2 | |
| Molar mass | 202.37 g·mol−1 |
| Appearance | Pale yellow or yellow powder |
| Density | 1.073 g/cm3 |
| Soluble | |
| Hazards | |
| GHS labelling: | |
   | |
| Warning | |
| H228, H302, H312, H315, H319, H335, H411 | |
| P210, P240, P241, P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P370+P378, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Production and properties
As typical for xanthates, potassium amyl xanthate is prepared by reacting n-amyl alcohol with carbon disulfide and potassium hydroxide.[1]
- CH3(CH2)4OH + CS2 + KOH → CH3(CH2)4OCS2K + H2O
Potassium amyl xanthate is a pale yellow powder. Its solutions are relatively stable between pH 8 and 13 with a maximum of stability at pH 10.[2]
Related compounds
- Sodium amyl xanthate is used in the separation of nickel and copper from their ores.[3]
References
- Charles C. Price and Gardner W. Stacy (1948). "p-nitrophenyl sulfide". Organic Syntheses. 28: 82.; Collective Volume, vol. 3, p. 667
- J. Dyer, L. H. Phifer, Macromolecules 2 (1969) 111. R. J. Millican, C. K. Sauers, J. Org. Chem. 44 (1979) 1964.
- Kerfoot, Derek G. E. (2005). "Nickel". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_157.
- Kathrin-Maria Roy "Xanthates" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.