Potassium hydrosulfide
Potassium hydrosulfide is the inorganic compound with the formula KSH. This colourless salt consists of the cation K+ and the bisulfide anion [SH]−. It is the product of the half-neutralization of hydrogen sulfide with potassium hydroxide. The compound is used in the synthesis of some organosulfur compounds.[1] Aqueous solutions of potassium sulfide consist of a mixture of potassium hydrosulfide and potassium hydroxide.
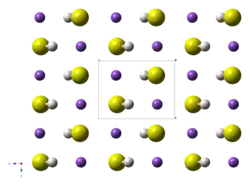 | |
| Names | |
|---|---|
| IUPAC name
Potassium hydrosulfide | |
| Other names
Potassium bisulfide, Potassium sulfhydrate, potassium hydrogen sulfide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.803 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| KSH | |
| Molar mass | 72.171 g/mol |
| Appearance | white solid |
| Density | 1.68–1.70 g/cm3 |
| Melting point | 455 °C (851 °F; 728 K) |
| good | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Flammable solid, stench, releases hydrogen sulfide |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions |
Potassium hydroxide |
Other cations |
Sodium hydrosulfide |
Related compounds |
potassium sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The structure of the potassium hydrosulfide resembles that for potassium chloride. Their structure is however complicated by the non-spherical symmetry of the SH− anions, but these tumble rapidly in the solid.[2]
Addition of sulfur gives dipotassium pentasulfide.
References
- Dittmer, Donald C. (2001). "Potassium Hydrogen Sulfide". In Paquette, L. (ed.). Encyclopedia of Reagents for Organic Synthesis. J. Wiley & Sons, New York. doi:10.1002/047084289X.rp227. ISBN 0471936235.
- Haarmann, F; Jacobs, H.; Roessler, E.; Senker, J. (2002). "Dynamics of Anions and Cations in Hydrogensulfides of Alkali Metals (NaHS, KHS, RbHS): A Proton Nuclear Magnetic Resonance Study". Journal of Chemical Physics. 117 (3): 1269–1276. Bibcode:2002JChPh.117.1269H. doi:10.1063/1.1483860.
- Kurzer, F.; Lawson, A. (1962). "Thiobenzoylthioglycolic Acid". Organic Syntheses. 42: 100. doi:10.15227/orgsyn.042.0100.
- Robert L. Frank and James R. Blegen (1948). "Benzoyl Disulfide". Organic Syntheses. 28: 16. doi:10.15227/orgsyn.028.0016.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
