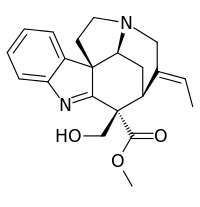
Preakuammicine
Preakuammicine is a terpene indole alkaloid.[2] Preakuammicine is thought to be formed from 4,21-dehydrogeissoschizine and lead to synthesis of stemmadenine. The enzymes involved in preakuammicine formation and those which use it as a substrate are currently unknown.
 | |
| Names | |
|---|---|
| IUPAC name
Methyl (16α,19E)-16-(hydroxymethyl)-1,2-didehydrocur-19-en-17-oate | |
| Other names
methyl (11S,12E,17S)-12-ethylidene-10-(hydroxymethyl)-8,14-diazapentacyclo[9.5.2.01,9.02,7.014,17]octadeca-2,4,6,8-tetraene-10-carboxylate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C21H24N2O3 | |
| Molar mass | 352.434 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- "Preakuammicine | Chemical Substance Information". J-GLOBAL.
- Benayad, Sarah; Ahamada, Kadiria; Lewin, Guy; Evanno, Laurent; Poupon, Erwan (March 2016). "Preakuammicine: A Long-Awaited Missing Link in the Biosynthesis of Monoterpene Indole Alkaloids". European Journal of Organic Chemistry. 2016 (8): 1494–1499. doi:10.1002/ejoc.201600102.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.