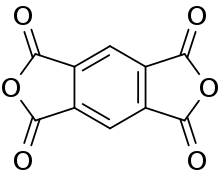
Pyromellitic dianhydride
Pyromellitic dianhydride (PMDA) is an organic compound with the formula C6H2(C2O3)2. It is the double carboxylic acid anhydride that is used in the preparation of polyimide polymers such as Kapton. It is a white, hygroscopic solid. It forms a hydrate.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1H,3H-Benzo[1,2-c:4,5-c′]difuran-1,3,5,7-tetrone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.001.726 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H2O6 | |
| Molar mass | 218.120 g·mol−1 |
| Appearance | White solid |
| Density | 1.68 g/cm3 |
| Melting point | 283 to 286 °C (541 to 547 °F; 556 to 559 K) |
| Boiling point | 397 to 400 °C (747 to 752 °F; 670 to 673 K) |
| Hygroscopic | |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H317, H318, H334 | |
| P261, P272, P280, P285, P302+P352, P304+P341, P305+P351+P338, P310, P321, P333+P313, P342+P311, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
It is prepared by gas-phase oxidation of 1,2,4,5-tetramethylbenzene (or related tetrasubstituted benzene derivatives). An idealized equation is:[1]
- C6H2(CH3)4 + 6 O2 → C6H2(C2O3)2 + 6 H2O
In the laboratory, it can be prepared by dehydration of pyromellitic acid using acetic anhydride.[2]
Reactions

PMDA is an electron-acceptor, forming a variety of charge-transfer complexes. It reacts with amines to diimides, C6H2[(CO)2NR]2 which also have acceptor properties.[4]
Applications
PMDA is used in PET bottle recycling as a chain extender. It increases the molecular weight of the polymer by linking-together alcohol and carboxylic acid groups formed by hydrolysis of the PET. This improves the rheological properties and overall quality of the recycled plastic.[5]
Safety
Evidence suggests that PMDA causes occupational asthma.[6]
References
- F. Röhrscheid (2012). "Carboxylic Acids, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_249.
- E. Philippi; R. Thelen (1930). "Pyromellitic Acid". Organic Syntheses. 10: 90. doi:10.15227/orgsyn.010.0090.
- Robertson, B. E.; Stezowski, J. J. (1978). "The crystal structure of the π-molecular complex of anthracene with pyromellitic dianhydride at –120°C". Acta Crystallographica Section B. 34 (10): 3005–3011. doi:10.1107/S0567740878009929.
- Song, Zhiping; Zhan, Hui; Zhou, Yunhong (2010). "Polyimides: Promising Energy-Storage Materials". Angewandte Chemie International Edition. 49 (45): 8444–8448. doi:10.1002/anie.201002439. PMID 20862664.
- Incarnato, L; Scarfato, P; Di Maio, L; Acierno, D (August 2000). "Structure and rheology of recycled PET modified by reactive extrusion". Polymer. 41 (18): 6825–6831. doi:10.1016/S0032-3861(00)00032-X.
- Madsen, Milene Torp; Skadhauge, Lars Rauff; Nielsen, Anders Daldorph; Baelum, Jesper; Sherson, David Lee (2019). "Pyromellitic dianhydride (PMDA) may cause occupational asthma". Occupational and Environmental Medicine. 76 (3): 175–177. doi:10.1136/oemed-2018-105295. PMC 6581108. PMID 30635433.