Pyrosulfate
In chemistry, disulfate or pyrosulfate is the anion with the molecular formula S
2O2−
7. Disulfate is the IUPAC name. [1]
It has a dichromate-like structure and can be visualised as two corner-sharing SO4 tetrahedra, with a bridging oxygen atom.[2]
In this anion, sulfur has an oxidation state of +6. Disulfate is the conjugate base of the hydrogen disulfate (hydrogen pyrosulfate) ion HS
2O−
7, which in turn is the conjugate base of disulfuric acid (pyrosulfuric acid).
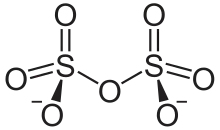
Chemical structure of the disulfate anion
References
- International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 130. Electronic version.
- Ståhl, K.; Balic-Zunic, T.; da Silva, F.; Eriksen, K.M.; Berg, R.W.; Fehrmann, R. (October 2005). "The crystal structure determinations and refinements of K2S2O7, KNaS2O7 and Na2S2O7 from X-ray powder and single crystal diffraction data". Journal of Solid State Chemistry. 178 (10): 1697–1704. Bibcode:2005JSSCh.178.1697S. doi:10.1016/j.jssc.2005.03.022.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.