Pyrrolidine alkaloids
The pyrrolidine alkaloids are natural products chemically derived from pyrrolidine.[1]
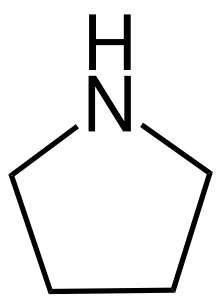
pyrrolidine, the fundamental chemical structure of pyrrolidine alkaloids.
Occurrence
Alkaloids with partial pyrrolidine structure are usually sub-categorized based on their occurrence and biogenetic origin. Hygrin and cuscohygrin were isolated from the leaves of the coca shrub,[2] while (-)-codonopsinine was isolated from the woodland vine tiger bell.[3]
 Coca shrub (Erythroxylum coca) with leaves and fruits.
Coca shrub (Erythroxylum coca) with leaves and fruits. Wood vine tiger bell (Codonopsis clematidea)
Wood vine tiger bell (Codonopsis clematidea)
Representatives
Among the most important representatives of the pyrrolidine alkaloids are hygrin and cuscohygrin.[2] Another representative is the (-)-codonopsinine.[3] Furthermore, ruspolinone, norruspolinone and norruspoline also belong to this alkaloid group.[4]
-Hygrine_Structural_Formula_V2.svg.png.webp) (+)-Hygrine
(+)-Hygrine-Codonopsinine_Structural_Formula_V2.svg.png.webp) (-)-Codonopsinine
(-)-Codonopsinine-Ruspolinone_Structural_Formula_V1.svg.png.webp) (-)-Ruspolinone
(-)-Ruspolinone-Norruspoline_Structural_Formula_V1.svg.png.webp) (R)-Norruspoline
(R)-Norruspoline
Properties
Many plants containing cuscohygrin are used in the folk medicine of various peoples as sedatives or narcotics.[5]
References
- Entry on Pyrrolidin. at: Römpp Online. Georg Thieme Verlag, retrieved {{{Datum}}}.
- H. Latscha, U. Kazmaier (2016), Chemie für Biologen (4 ed.), Berlin Heidelberg: Springer Spektrum, p. 682, ISBN 978-3-662-47783-0
- J. Reddy, B. Rao (2007), "A Short, Efficient, and Stereoselective Total Synthesis of a Pyrrolidine Alkaloid: (−)-Codonopsinine", The Journal of Organic Chemistry, vol. 72, no. 6, pp. 2224–2227, doi:10.1021/jo061940q, PMID 17316046
- F. Roessler, D. Ganzinger, S. Johne, E.Schöpp, M. Hesse (1978), "Ruspolia hypercrateriformis M.R.:Isolierung und Strukturaufklärung von neuen Pyrrolidin‐Alkaloiden. 169. Mitt. über organische Naturstoffe", Helvetica Chimica Acta, vol. 61, no. 3, pp. 1200–1206, doi:10.1002/hlca.19780610336
{{citation}}: CS1 maint: multiple names: authors list (link) - Entry on Cuscohygrin. at: Römpp Online. Georg Thieme Verlag, retrieved {{{Datum}}}.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.