Quassinoid
Quassinoids are degraded triterpene lactones (similar to limonoids) of the Simaroubaceae plant family grouped into C-18, C-19, C-20, C-22 and C-25 types.[1] The prototypical member of the group, quassin, was first described in the 19th century from plants of the genus Quassia from which it gets its name.[2] It was isolated in 1937 and its structure elucidated in 1961.
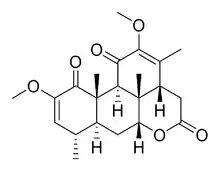
Chemical structure of quassin
They are a biologically potent class of natural products, possessing antimalarial,[3] antifeedant,[4] insecticidal,[5] anti-inflammatory,[6] and anticancer[7] properties. The quassinoid bruceantin reached two separate phase II clinical trials in 1982[8] and 1983.[9]
Other quassinoids include:[10]
- Bruceanols
- Bruceolide
- Eurycomanone
- Gutolactone
- Isobrucein A
- Neoquassin
- Nigakihemiacetal A
- Quassimarin
- Samaderines
- Simalikalactones
References
- Vieira, Studies in Natural Products Chemistry 2006 https://www.researchgate.net/profile/Ivo_Vieira/publication/251467805
- Winckler, F. L. (1835). Rep. Pharm. 4: 85.
{{cite journal}}: Missing or empty|title=(help) - Muhammad, I.; Samoylenko, V. (2007). "Antimalarial quassinoids: Past, present and future". Expert Opin Drug Discov. 2 (8): 1065–84. doi:10.1517/17460441.2.8.1065. PMID 23484873. S2CID 24497786.
- Leskinen, V.; Polonsky, J.; Bhatnagar, S. (1984). "Antifeedant activity of quassinoids". J. Chem. Ecol. 10 (10): 1497–507. doi:10.1007/BF00990319. PMID 24318349. S2CID 21117128.
- Fang, X.; Di, Y. T.; Xhang, Y.; Xu, Z. P.; Lu, Y.; Chen, Q. Q.; Zheng, Q. T.; Hao, X. J. (2015). "Unprecedented Quassinoids with Promising Biological Activity from Harrisonia perforata". Angew. Chem. Int. Ed. 54 (19): 5592–5. doi:10.1002/anie.201412126. PMID 25810025.
- Hall, I. H.; Lee, K. H.; Imakura, Y.; Okano, M.; Johnson, A. (1983). "Anti-inflammatory Agents III: Structure–Activity Relationships of Brusatol and Related Quassinoids". J. Pharm. Sci. 72 (11): 1282–4. doi:10.1002/jps.2600721111. PMID 6417321.
- Fukamiya, N.; Lee, K.H.; Muhammad, I., Murakami, C.; Okano, M.; Harvey, I.; Pelletier, J. (2005). "Structure–activity relationships of quassinoids for eukaryotic protein synthesis". Cancer Letters. 220 (1): 37–48. doi:10.1016/j.canlet.2004.04.023. PMID 15737686.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Wiseman, C. L.; Yap, H. Y.; Bedikian, A. Y.; Bodey, G. P.; Blumenchein, G. R. (1982). "Phase II trial of bruceantin in metastatic breast carcinoma". Am. J. Clin. Oncol. 5 (4): 389–91. doi:10.1097/00000421-198208000-00007. PMID 7113961. S2CID 27632884.
- Arsenau, J. C.; Wolter, J. M.; Kuperminc, M.; Ruckdeschel, J. C. (1983). "Anti–inflammatory agents III: Structure–activity relationships of brusatol and related quassinoids". Invest. New Drugs. 1: 239.
- "Quassinoid". Chemical Entities of Biological Interest (ChEBI).
External links
 Media related to quassinoids at Wikimedia Commons
Media related to quassinoids at Wikimedia Commons The dictionary definition of quassinoid at Wiktionary
The dictionary definition of quassinoid at Wiktionary
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.