Isoetes
Isoetes, commonly known as the quillworts, is a genus of lycopod. It is the only living genus in the family Isoetaceae and order Isoetales. There are currently 192 recognized species,[1] with a cosmopolitan distribution mostly in aquatic habitats but with the individual species often scarce to rare. Some botanists split the genus, separating two South American species into the genus Stylites, although molecular data place these species among other species of Isoetes, so that Stylites does not warrant taxonomic recognition.[2] Species virtually identical to modern quillworts have existed since the Jurassic epoch,[3] though the timing of the origin of modern Isoetes is subject to considerable uncertainty.[4]
| Isoetes Temporal range: | |
|---|---|
 | |
| Isoetes tegetiformans with U.S. penny for scale | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Lycophytes |
| Class: | Lycopodiopsida |
| Order: | Isoetales |
| Family: | Isoetaceae |
| Genus: | Isoetes L. |
| Species | |
|
See text | |
The name of the genus may also be spelled Isoëtes. The diaeresis (two dots over the e) indicates that the o and the e are to be pronounced in two distinct syllables. Including this in print is optional; either spelling (Isoetes or Isoëtes) is correct.[5]
Description
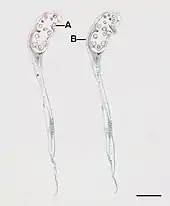
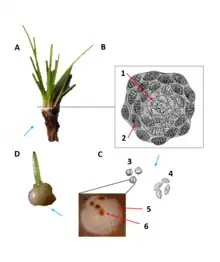
Quillworts are mostly aquatic or semi-aquatic in clear ponds and slow-moving streams, though several (e.g. I. butleri, I. histrix and I. nuttallii) grow on wet ground that dries out in the summer. The quillworts are spore-producing plants and highly reliant on water dispersion. Quillworts have different ways to spread their spores based on the environment. Quillwort leaves are hollow and quill-like, with a minute ligule at the base of the upper surface.[6]: 7 arising from a central corm. The sporangia are sunk deeply in the leaf bases. Each leaf will either have many small spores or fewer large spores. Both types of leaf are found on each plant.[7] Each leaf is narrow, 2–20 centimetres (0.8–8 in) long (exceptionally up to 100 cm or 40 in) and 0.5–3.0 mm (0.02–0.12 in) wide; they can be either evergreen, winter deciduous, or dry-season deciduous. Only 4% of total biomass, the tips of the leaves, is chlorophyllous.[8]
The roots broaden to a swollen base up to 5 mm (0.2 in) wide where they attach in clusters to a bulb-like, underground rhizome characteristic of most quillwort species, though a few (e.g. I. tegetiformans) form spreading mats. This swollen base also contains male and female sporangia, protected by a thin, transparent covering (velum), which is used diagnostically to help identify quillwort species. They are heterosporous. Quillwort species are very difficult to distinguish by general appearance. The best way to identify them is by examining their megaspores under a microscope. Moreover, habitat, texture, spore size, and velum provide features that distinguish Isoëtes taxa.[9] They also possess a vestigial form of secondary growth in the basal portions of its cormlike stem, an indication that they evolved from larger ancestors.[10]
Biochemistry and genetics
Quillworts use Crassulacean acid metabolism (CAM) for carbon fixation. Some aquatic species don't have stomata and the leaves have a thick cuticle which prevents CO2 uptake, a task that is performed by their hollow roots instead, which absorb CO2 from the sediment.[11] This has been studied extensively in Isoetes andicola.[8] CAM is normally considered an adaptation to life in arid environments to prevent water loss with the plants opening their stomata at night rather than in the heat of the day. This allows CO2 to enter and minimises water loss. As mostly submerged aquatic plants, quillworts do not lack water and the use of CAM is considered to avoid competition with other aquatic plants for CO2 during daytime.[12]
The first detailed quillwort genome sequence, of I. taiwanensis,[13] showed that there were differences from CAM in terrestrial plants. CAM involves the enzyme phosphoenolpyruvate carboxylase (PEPC) and plants have two forms of the enzyme. One is normally involved in photosynthesis and the other in central metabolism. From the genome sequence, it appears that in quillworts, both forms are involved in photosynthesis. In addition, the time of day of the peak abundance of some of the components of CAM was different from terrestrial plants. These fundamental differences in biochemistry suggests that CAM in quillworts is probably another example of convergent evolution of CAM during the more than 300 million years since the genus diverged from other plants. However, they may also be because of differences between life in water and in the air.[13] The genome sequence also provided two insights into its structure. First, genes and repeated non-coding regions were fairly evenly distributed across all the chromosomes. This is similar to genomes of other non-seed plants, but different from the seed plants (angiosperms) where there are distinctly more genes at the ends of chromosomes. Secondly, there was also evidence that the whole genome had been duplicated in the ancient past.[13]
Reproduction
Overview
Like all land plants, Isoetes undergoes an alternation of generations between a diploid sporophyte stage and a sexual haploid gametophyte stage. However, the dominance of one stage over the other has shifted over time. The development of vascular tissue and subsequent diversification of land plants coincides with the increased dominance of the sporophyte and reduction of the gametophyte. Isoetes, as members of the Lycopodiopsida class, are part of the oldest extant lineage that reflects this shift to a sporophyte dominant lifecycle. In closely related lineages, such as the extinct Lepidodendron, spores were dispersed by the sporophyte through large collections of sporangia called strobili for wind-based spore dispersal.[14] However, Isoetes are small heterosporous semi-aquatic plants, with different reproductive needs and challenges than large tree-like land plants.
Description
Like the rest of the Lycopodiopsida class, Isoetes reproduces with spores.[15] Among the lycophytes, both Isoetes and the Selaginellaceae (spikemosses) are heterosporous, while the remaining lycophyte family Lycopodiaceae (clubmosses) is homosporous.[16] As heterosporous plants, fertile Isoetes sporophytes produce megaspores and microspores, which develop in the megasporangia and microsporangia.[17] These spores are highly ornate and are the primary way by which species are identified, although no one functional purpose of the intricate surface patterns is agreed upon.[18] The megasporangia occur within the outermost microphylls (single-veined leaves) of the plant while the microsporangia are found in the innermost microphylls.[19] This pattern of development is hypothesized to improve the dispersal of the heavier megaspore.[15] These spores then germinate and divide into mega- and micro- gametophytes.[17][20][21] The microgametophytes have antheridia, which in turn produce sperm.[21] The megagametophytes have archegonia, which produce egg cells.[21] Fertilization takes place when the motile sperm from a microgametophyte locates the archegonia of a megagametophyte and swims inside to fertilize the egg.
Outside of heterospory, a distinguishing feature of Isoetes (and Selaginella) from other pteridophytes, is that their gametophytes grow inside the spores.[17][21][19] This means that the gametophytes never leave the protection of the spore that disperses them, cracking the perispore (the outer layer of the spore) just enough to allow the passage of gametes. This is fundamentally different from ferns, where the gametophyte is a photosynthetic plant exposed to the elements of its environment. However, containment creates a separate problem for Isoetes, which is that the gametophytes have no way to acquire energy on their own. Isoetes sporophytes solve this problem by provisioning starches and other nutrients to the spores as an energy reserve for the eventual gametophytes.[21][22] Although not a homologous process, this provisioning is somewhat analogous to other modes of offspring resource investment in seed-plants, such as fruits and seeds. The extent to which resources provisioned to the megaspore also support the growth of the new sporophyte is unknown in Isoetes.
Dispersal
Spore dispersal occurs primarily in water (hydrochory) but may also occur via adherence to animals (zoochory) and as a result of ingestion (endozoochory).[15][23] These are among the reasons suggested for the ornamentations of the spore, with some authors demonstrating that certain patterns seem well-adapted for sticking to relevant animals like waterfowl.[23] Another critical element of dispersal is the observation that in some species of Isoetes, the outer coat of megaspores have pockets that trap microspores, a condition known as synaptospory.[23][24] Typically, heterospory means that colonization and long-dispersal are more difficult due to the fact that a single spore cannot grow a bisexual gametophyte and thus cannot establish a new population from a single spore as can happen in homosporous ferns.[25] Isoetes may mitigate this issue via microspores stuck to megaspores, greatly increasing the possibility of successful fertilization upon dispersal.[23][24]
Taxonomy
Compared to other genera, Isoetes is poorly known. The first critical monograph on their taxonomy, written by Norma Etta Pfeiffer, was published in 1922 and remained a standard reference into the twenty-first century.[26][27] Even after studies with cytology, scanning electron microscopy, and chromatography, species are difficult to identify and their phylogeny is disputed. Vegetative characteristics commonly used to distinguish other genera, such as leaf length, rigidity, color, or shape are variable and depend on the habitat. Most classification systems for Isoetes rely on spore characteristics, which make species identification nearly impossible without microscopy.[28]
Evolution
The earliest fossil that has been assigned to the genus is †Isoetes beestonii from the latest Permian[29] of New South Wales, Australia, around 252 million years ago.[30] However, the relationships of pre-Jurassic isoetaleans to modern Isotetes have been regarded as unclear by other authors.[3] Isoetites rolandii from the Late Jurassic of North America has been described as the "earliest clear example of a isoetalean lycopsid containing all the major features uniting modern Isoetes", including the loss of the elongated stem and vegetative leaves. Based on this, it has been stated that "the overall morphology of Isoetes appears to have persisted virtually unchanged since at least the Jurassic".[3] The timing of the origin of the crown group is uncertain. Wood et al (2020) asserted there to be no morphological features that define the major clades within Isoetes, and no fossils are known that can be definitively assigned to the crown group.[3] While Wood et al. suggested a young origin dating to the early Cenozoic based on molecular clock estimates[3], the results were questioned by Wikström et al. (2023) who regarded the molecular clock as providing no firm evidence for the origin time of the genus, which could date to the Mesozoic or even the late Paleozoic, depending on the calibration method used.[4]
Extant species
As of November 2019, Plants of the World Online accepted the following extant species:[31]
- I. abyssinica Chiov.
- I. acadiensis Kott
- I. aemulans J.P.Roux
- I. aequinoctialis Welw. ex A.Br.
- I. alcalophila S.Halloy
- I. alpina Kirk
- I. alstonii C.F.Reed & Verdc.
- I. amazonica A.Br.
- I. anatolica Prada & Rolleri
- I. andicola (Amstutz) L.D.Gómez
- I. andina Spruce ex Hook.
- I. appalachiana D.F.Brunt. & D.M.Britton
- I. araucaniana Macluf & Hickey
- I. asiatica (Makino) Makino
- I. attenuata C.R.Marsden & Chinnock
- I. australis S.Williams
- I. azorica Durieu
- I. baculata Hickey & H.P.Fuchs
- I. biafrana Alston
- I. bischlerae H.P.Fuchs
- I. bolanderi Engelm.
- I. boliviensis U.Weber
- I. boomii Luebke
- I. boryana Durieu
- I. boyacensis H.P.Fuchs
- I. bradei Herter
- I. brasiliensis H.P.Fuchs
- I. brevicula E.R.L.Johnson
- I. butleri Engelm.
- I. cangae J.B.S.Pereira, Salino & Stützel
- I. capensis
- I. caroli E.R.L.Johnson
- I. caroliniana (A.A.Eaton) Luebke is regarded by Plants of the World Online as a synonym of I. valida, but other sources treat it as a valid species[32]
- I. chubutiana Hickey, Macluf & W.C.Taylor
- I. coromandelina L.f.
- I. creussensis Lazare & S.Riba
- I. cristata C.R.Marsden & Chinnock
- I. cubana Engelm.
- I. delilei (Bory) Rothm.
- I. dispora Hickey
- I. dixitii Shende
- I. drummondii A.Braun
- I. durieui Bory
- I. echinospora Durieu
- I. ecuadoriensis Aspl.
- I. ekmanii U.Weber
- I. elatior A.Braun
- I. eludens J.P.Roux, Hopper & Rhian J.Sm.
- I. engelmannii A.Braun
- I. escondidensis S.Halloy
- I. eshbaughii Hickey & H.P.Fuchs
- I. flaccida Shuttlew.
- I. fluitans M.I.Romero
- I. fuliginosa R.L.Small & Hickey
- I. fuscomarginata H.P.Fuchs
- I. gardneriana Kunze
- I. georgiana Luebke
- I. giessii Launert
- I. gigantea U.Weber
- I. graniticola D.F.Brunt.
- I. gunnii A.Braun
- I. gymnocarpa (Gennari) A.Braun
- I. habbemensis Alston
- I. hallasanensis H.K.Choi, Ch.Kim & J.Jung
- I. haussknechtii Troìa & Greuter
- I. hawaiiensis W.C.Taylor & W.H.Wagner
- I. heldreichii Wettst.
- I. hemivelata R.L.Small & Hickey
- I. herzogii U.Weber
- I. hewitsonii Hickey
- I. hieronymi U.Weber
- I. histrix Bory
- I. hopei J.R.Croft
- I. howellii Engelm.
- I. humilior A.Braun
- I. hypsophila Hand.-Mazz.
- I. inflata E.R.L.Johnson
- I. jaegeri Pitot
- I. jamaicensis Hickey
- I. japonica A.Braun
- I. jejuensis H.K.Choi, Ch.Kim & J.Jung
- I. junciformis D.F.Brunt. & D.M.Britton
- I. karstenii A.Braun
- I. killipii C.V.Morton
- I. kirkii A.Braun
- I. labri-draconis N.R.Crouch
- I. lacustris L.
- I. laosiensis C.Kim & H.K.Choi
- I. lechleri Mett.
- I. libanotica Musselman, Bolin & R.D.Bray
- I. lithophila N.Pfeiff.
- I. longissima Bory
- I. louisianensis Thieret
- I. luetzelburgii U.Weber
- I. macrospora
- I. malinverniana Ces. & De Not.
- I. maritima Underw. – maritime quillwort
- I. martii A.Braun
- I. mattaponica Musselman & W.C.Taylor
- I. maxima Hickey, Macluf & Link-Pérez
- I. melanopoda J.Gay & Durieu (I. virginica N.Pfeiff.)
- I. melanospora Engelm.
- I. melanotheca Alston
- I. mexicana Underw. (syn. Isoetes montezumae A.A.Eaton)
- I. microvela D.F.Brunt.
- I. minima A.A.Eaton
- I. mississippiensis S.W.Leonard, et al.
- I. mongerensis E.R.L.Johnson
- I. montana U.Weber
- I. mourabaptistae J.B.S.Pereira,et al.
- I. muelleri A.Braun
- I. naipiana P.G.Windisch, Lorscheitt. & Nervo
- I. nana J.B.S.Pereira
- I. neoguineensis
- I. nigritiana A.Br.
- I. nigroreticulata Verdc.
- I. novogranadensis H.P.Fuchs
- I. nuttallii A.Braun
- I. occidentalis L.F.Hend.
- I. olympica A.Br.
- I. orcuttii A.A.Eaton
- I. organensis U.Weber
- I. orientalis Hong Liu & Q.F.Wang
- I. ovata N.Pfeiff.
- I. pallida Hickey
- I. palmeri H.P.Fuchs
- I. panamensis Maxon & C.V.Morton
- I. parvula Hickey
- I. pedersenii H.P.Fuchs ex E.I.Meza & Macluf
- I. perralderiana Durieu & Letourn. ex Milde
- I. perrieriana Iversen
- I. philippinensis Merr. & L.M.Perry
- I. phrygia Hausskn.
- I. piedmontana (N.Pfeiff.) C.F.Reed
- I. pitotii Alston
- I. precocia R.L.Small & Hickey
- I. pringlei Underw.
- I. prototypus D.M.Britton & Goltz
- I. pseudojaponica M.Takamiya, Mits.Watan. & K.Ono
- I. pusilla C.R.Marsden & Chinnock
- I. quiririensis J.B.S.Pereira & Labiak
- I. ramboi Herter
- I. riparia Engelm. ex A.Braun (syn I. hyemalis D.F.Brunt.)
- I. sabatina Troìa & Azzella
- I. saccharata Engelm.
- I. sahyadrii Mahab.
- I. saracochensis Hickey
- I. savatieri Franch.
- I. schweinfurthii A.Br.
- I. sehnemii H.P.Fuchs
- I. septentrionalis D.F.Brunt.
- I. serracarajensis J.B.S.Pereira, Salino & Stützel
- I. setacea Lam.
- I. sinensis T.C.Palmer (synonym I. coreana Y.H.Chung & H.K.Choi)
- I. smithii H.P.Fuchs
- I. spannagelii H.P.Fuchs
- I. spinulospora C.Jermy & Schelpe
- I. stellenbossiensis A.V.Duthie
- I. stephanseniae A.V.Duthie
- I. stevensii J.R.Croft
- I. storkii T.C.Palmer
- I. taiwanensis De Vol
- I. tamaulipana Mora-Olivo, A.Mend. & Mart.-Aval.
- I. tegetiformans Rury
- I. tenella Léman ex Desv.
- I. tennesseensis Luebke & Budke
- I. tenuifolia Jermy
- I. tenuissima Boreau
- I. texana Singhurst, Rushing & W.C.Holmes
- I. todaroana Troìa & Raimondo
- I. toximontana Musselman & J.P.Roux
- I. transvaalensis C.Jermy & Schelpe
- I. triangula U.Weber
- I. tripus A.Braun
- I. truncata Clute
- I. tuckermanii A.Braun ex Engelm.
- I. tuerckheimii Brause
- I. udupiensis P.K.Shukla, G.K.Srivast., S.K.Shukla & P.K.Rajagopal
- I. ulei U.Weber
- I. valida Clute
- I. vanensis M.Keskin & G.Zare
- I. vermiculata Hickey
- I. viridimontana M.A.Rosenthal & W.C.Taylor
- I. weberi Herter
- I. welwitschii A.Br. ex Kuhn
- I. wormaldii Sim
- I. yunguiensis Q.F.Wang & W.C.Taylor
Many species, such as the Louisiana quillwort and the mat-forming quillwort, are endangered species. Several species of Isoetes are commonly called Merlin's grass, especially I. lacustris, but also the endangered species I. tegetiformans.
Hybrids
- I. × altonharvillii Musselman
- I. × brittonii D.F.Brunt. & W.C.Taylor
- I. × bruntonii Knepper & Musselman
- I. × carltaylorii Musselman
- I. × dodgei A.A.Eaton
- I. × eatonii R.Dodge – Eaton's quillwort
- I. × echtuckerii D.F.Brunt. & D.M.Britton
- I. × fairbrothersii J.D.Montgom. & W.C.Taylor
- I. × foveolata A.A.Eaton
- I. × gopalkrishnae S.K.Singh, P.K.Shukla & N.K.Dubey
- I. × harveyi A.A.Eaton (syn. I. × heterospora Eaton)
- I. × herb-wagneri W.C.Taylor
- I. × hickeyi W.C.Taylor & Luebke
- I. × jeffreyi D.M.Britton & D.F.Brunt.
- I. × marensis D.M.Britton & D.F.Brunt.
- I. × michinokuana M.Takamiya, Mits.Watan. & K.Ono
- I. × novae-angliae D.F.Brunt. & D.M.Britton
- I. × paratunica D.F.Brunt., Mochalova & A.A.Bobrov
- I. × pseudotruncata D.M.Britton & D.F.Brunt.
References
- Troia, Angelo; Pereira, Jovani B.; Kim, Changkyun; Taylor, W. Carl (2016). "The genus Isoetes (Isoetaceae): a provisional checklist of the accepted and unresolved taxa". Phytotaxa. 277 (2): 101. doi:10.11646/phytotaxa.277.2.1. ISSN 1179-3163.
- Larsén, Eva; Rydin, Catarina (2016). "Disentangling the Phylogeny of Isoetes (Isoetales), Using Nuclear and Plastid Data". International Journal of Plant Sciences. 177 (2): 157–174. doi:10.1086/684179. ISSN 1058-5893. S2CID 85737029.
- Wood, Daniel; Besnard, Guillaume; Beerling, David J.; Osborne, Colin P.; Christin, Pascal-Antoine (18 June 2020). "Phylogenomics indicates the "living fossil" Isoetes diversified in the Cenozoic". PLOS ONE. 15 (6): e0227525. Bibcode:2020PLoSO..1527525W. doi:10.1371/journal.pone.0227525. ISSN 1932-6203. PMC 7302493. PMID 32555586.
- Wikström, Niklas; Larsén, Eva; Khodabandeh, Anbar; Rydin, Catarina (January 2023). "No phylogenomic support for a Cenozoic origin of the "living fossil" Isoetes". American Journal of Botany. 110 (1): e16108. doi:10.1002/ajb2.16108. ISSN 0002-9122. PMC 10108322. PMID 36401556.
- International Code of Nomenclature for algae, fungi, and plants (Melbourne Code) see section 60.6: "The diaeresis, indicating that a vowel is to be pronounced separately from the preceding vowel (as in Cephaëlis, Isoëtes), is a phonetic device that is not considered to alter the spelling; as such, its use is optional."
- Stace, C. A. (2010). New Flora of the British Isles (3rd ed.). Cambridge, U.K.: Cambridge University Press. ISBN 9780521707725.
- Levyns, M.R. (1966). A Guide to the Flora of the Cape Peninsula (2nd Revised ed.). Juta & Company, Limited. OCLC 621340.
- Tropical Alpine Environments: Plant Form and Function
- Isoëtes Linnaeus, Sp. Pl. 2: 1100. 1753; Gen. Pl. ed. 5, 486, 1754.
- The Formation of Wood in Forest Trees: The Second Symposium Held under the Auspices of the Maria Moors Cabot Foundation for Botanical Research
- Jacobsen, Dean; Dangles, Olivier (18 August 2017). Ecology of High Altitude Waters]. Oxford University Press. ISBN 9780191056666.
- Haas, Michael J. (2 December 2021). "Quillwort genome highlights divergences in aquatic CAM photosynthesis". The Global Plant Council. Retrieved 29 December 2021.
- Wickell, David; Kuo, Li-Yaung; Yang, Hsiao-Pei; others, and 11 (2021). "Underwater CAM photosynthesis elucidated by Isoetes genome". Nature Communications. 12 (1): 6348. Bibcode:2021NatCo..12.6348W. doi:10.1038/s41467-021-26644-7. PMC 8566536. PMID 34732722.
- Kenrick, Paul. (1997). The origin and early diversification of land plants : a cladistic study. Crane, Peter R. Washington, DC: Smithsonian Institution Press. ISBN 1-56098-730-8. OCLC 37107157.
- Taylor, W. Carl; Hickey, R. James (1992). "Habitat, Evolution, and Speciation in Isoetes". Annals of the Missouri Botanical Garden. 79 (3): 613. doi:10.2307/2399755. JSTOR 2399755.
- "A community-derived classification for extant lycophytes and ferns". Journal of Systematics and Evolution. 54 (6): 563–603. 2016. doi:10.1111/jse.12229. ISSN 1759-6831. S2CID 39980610.
- FARMER, J. BRETLAND (1890). "On Isoetes lacustris, L." Annals of Botany. 5 (17): 37–62. ISSN 0305-7364. JSTOR 43234433.
- Hickey, R. James (January 1986). "Isoetes Megaspore Surface Morphology: Nomenclature, Variation, and Systematic Importance". American Fern Journal. 76 (1): 1–16. doi:10.2307/1547394. ISSN 0002-8444. JSTOR 1547394.
- La Motte, Charles (April 1933). "Morphology of the Megagametophyte and the Embryo Sporophyte Ofisoetes Lithophila". American Journal of Botany. 20 (4): 217–233. doi:10.1002/j.1537-2197.1933.tb08887.x.
- SCOTT, D. H.; HILL, T. G. (1900). "The Structure of Isoetes Hystrix". Annals of Botany. 14 (55): 413–454. doi:10.1093/oxfordjournals.aob.a088787. ISSN 0305-7364. JSTOR 43235515.
- LA MOTTE, CHARLES (1937). "Morphology and Orientation of the Embryo of Isoetes". Annals of Botany. 1 (4): 695–715. doi:10.1093/oxfordjournals.aob.a083498. ISSN 0305-7364. JSTOR 42906582.
- Abeli, Thomas; Mucciarelli, Marco (2010). "Notes on the Natural History and Reproductive Biology of Isoëtes malinverniana". American Fern Journal. 100 (4): 235–237. doi:10.1640/0002-8444-100.4.235. ISSN 0002-8444. JSTOR 41237871. S2CID 83658338.
- Troia, Angelo (16 June 2016). "Dispersal and colonization in heterosporous lycophytes: palynological and biogeographical notes on the genusIsoetesin the Mediterranean region". Webbia. 71 (2): 277–281. doi:10.1080/00837792.2016.1191171. ISSN 0083-7792. S2CID 89179370.
- Lellinger, David B.; Kramer, K. U. (April 1979). "Synaptospory: A Hypothesis". American Fern Journal. 69 (2): 48. doi:10.2307/1546895. ISSN 0002-8444. JSTOR 1546895.
- Sessa, Emily B.; Testo, Weston L.; Watkins, James E. (20 April 2016). "On the widespread capacity for, and functional significance of, extreme inbreeding in ferns". New Phytologist. 211 (3): 1108–1119. doi:10.1111/nph.13985. ISSN 0028-646X. PMID 27094807.
- Pfeiffer, Norma E. (1922). "Monograph of the Isoetaceae". Annals of the Missouri Botanical Garden. 9 (2): 79 –. doi:10.2307/2990000. JSTOR 2990000. Retrieved 29 December 2021.
- Haas, Michael J. "Secrets of quillwort photosynthesis could boost crop efficiency". Cornell Chronicle. Retrieved 12 March 2023.
- Cody, William; Britton, Donald (1989). Ferns and Fern Allies of Canada. Agriculture Canada. ISBN 9780660131023.
- Retallack, G.J. (2013). "Permian and Triassic greenhouse crises". Gondwana Research. 24 (1): 90–103. Bibcode:2013GondR..24...90R. doi:10.1016/j.gr.2012.03.003.
- Retallack, G.J. (1997). "Earliest Triassic origin of Isoetes and quillwort evolutionary radiation". Journal of Paleontology. 71 (3): 500–521. Bibcode:1997JPal...71..500R. doi:10.1017/S0022336000039524. ISSN 0022-3360. S2CID 140566050.
- "Isoetes L.". Plants of the World Online. Royal Botanic Gardens, Kew. Retrieved 18 November 2019.
- Hassler, Michael & Schmitt, Bernd (November 2019). "Isoetes caroliniana". Checklist of Ferns and Lycophytes of the World. 8.11. Retrieved 18 November 2019.
- Pigg, K.B. (2001). "Isoetalean lycopsid evolution: from the Devonian to the present". American Fern Journal. 91 (3): 99–114. doi:10.1640/0002-8444(2001)091[0099:ILEFTD]2.0.CO;2. S2CID 85852292.
- Hill, R.S. (1988). "Tertiary Isoetes from Tasmania". Alcheringa. 12 (2): 157–162. doi:10.1080/03115518808619003.
- Britton, D. M. (1993). "Isoëtes reticulata RS Hill 1987 (Alcheringa 12: 158) is an illegitimate name". American Fern Journal. 83 (4): 128. doi:10.2307/1547589. JSTOR 1547589.