Radiofrequency Echographic Multi Spectrometry
Radiofrequency Echographic Multi Spectrometry (REMS) is a non-ionizing technology for osteoporosis diagnosis and for fracture risk assessment. REMS processes the raw, unfiltered ultrasound signals acquired during an echographic scan of the axial sites, femur and spine. The analysis is performed in the frequency domain. Bone mineral density (BMD) is estimated by comparing the results against reference models.
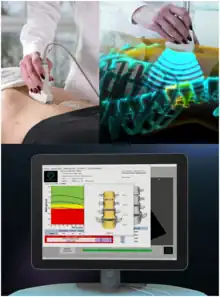
The accuracy has been tested by comparing it against to DXA technology.[1][2][3][4]
Working principles

Traditionally, ultrasound B-Mode imaging has been designed for allowing a visual evaluation of human organs and their features by clinicians; however, this implies that the huge quantity of information carried by ultrasound signals is processed and significantly reduced for visualization purposes. REMS technology instead analyses the raw, unfiltered ultrasound signals by comparing their spectral representation with the spectral models stored in a proprietary database which has been previously obtained from healthy and osteoporotic patients; these models are specific and vary with sex, age, BMI and skeletal site. The comparison allows the BMD estimation of the patient[5][6] as well as a both fast and reliable diagnostic classification, compliant to the recommendations and diagnostic criteria defined by the World Health Organization.
Spectral and statistical processing of the acquired data
REMS scans on femur and spine last 40 and 80 seconds, respectively, allowing the acquisition of several thousands of ultrasound signals related to the skeletal site under examination. The patented algorithm (see[5][6] for more details) automatically processes these signals on the basis of their spectral features; each signal can be classified as reliable and included in the pipeline for the computation of the diagnostic parameters or, alternatively, classified as unreliable and discarded. During the analysis phase, the acquired spectra are compared to the spectral models stored in the database; afterwards, the values obtained by each comparison are averaged, leading to a precise and repeatable estimation of the diagnostic parameters of interest.
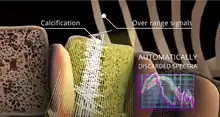
Automatic artifact removal
If substantial differences are detected between one or more acquired signals and the reference spectral models, these samples are identified, classified as unreliable and automatically discarded: for instance, spectra which are not clearly associated to bone portions but to osteophytes or calcifications. Hence, this approach natively identifies and eliminates outliers, bringing significant advantages with respect to the clinical reliability of the obtained results.[7][8][9]
Performance comparison to the current Gold Standard
REMS technology performance has been evaluated through multicentre clinical studies.[1][10] The work of Di Paola et al.[1] has investigated precision and diagnostic accuracy of REMS in comparison with DXA on a sample of 2000 patients. A very high correlation has been observed between the T-Score values obtained by both technologies (Pearson correlation coefficient > 0.93; Cohen’s Kappa equals to 0.82 for lumbar spine and 0.79 for femoral neck) as well as a very low average BMD difference between the two techniques (mean ± 2 standard deviations): −0.004±0.088 g/cm2 for lumbar spine and −0.006±0.076 g/cm2 for femoral neck. Furthermore, specificity and sensitivity of REMS in the discrimination between osteoporotic and non-osteoporotic patients has been evaluated: sensitivity and specificity exceed 91% for both skeletal sites. Additional outcomes of this study are the values of precision and repeatability of REMS estimates, assessed using the Root Mean Square Coefficient of Variation (CV-RMS): precision has been evaluated as being 0.38% for lumbar spine and 0.32% for femoral neck, whereas the Least Significant Change (LSC) resulted in 1.05% and 0.88%, respectively. Finally, inter-operator repeatability has been calculated, which has resulted in 0.54% for lumbar spine and 0.48% for femoral neck. These values are significantly lower than those reported about DXA in the scientific literature[11] and offer concrete advantages from the point of view of short-term follow-up of patients undergoing therapeutic treatments.[12][13]
Fracture risk evaluation
Observational longitudinal studies have further evaluated REMS T-score performance in the identification of patients at risk for fragility fracture.[1][2] Specifically, in Adami et al.,[1] a group of more than 1.500 patients has undergone both DXA and REMS scans. Afterwards, these patients have been monitored for a period up to 5 years in order to estimate the incidence of fragility fractures in relationship with the T-score values previously obtained with both technologies. The study has demonstrated that REMS T-score is an effective parameter for the prediction of the occurrence of fragility fractures, leading the authors to positive conclusions about the effectiveness of REMS technology in the identification of patients at risk for osteoporotic fracture.
REMS Technology and Fragility Score

As widely reported in the scientific literature,[14] bone density is just one of the components of bone strength thus it only partially predicts bone fragility. In order to overcome this limitation, a novel parameter, Fragility Score, has been developed. Fragility Score evaluates bone microstructural features independently from BMD and it is based on the assumption that a fragile bone structure has microstructural features which, in turn, influence the spectral characteristics of the acquired ultrasound signal, being different from those reflecting a robust bone structure. Fragility Score is an adimensional parameter, ranging from 0 to 100, obtained by comparing the spectra of the acquired ultrasound signals with the spectral reference models obtained from patients who did, or did not, developed an osteoporotic fracture. This parameter has been validated through clinical studies and its accuracy has demonstrated a performance similar to DXA BMD.[15][16]
International recognition and clinical use
In a recent publication, REMS technology has received the attention of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). In this work, all the available technologies for bone strength assessment and fracture risk estimation have been reviewed and discussed in relation to the clinical needs currently unmet.[14] In this context, REMS has been considered a valuable approach for osteoporosis diagnosis and for fracture risk assessment, at the same time overcoming several of the current limitations acknowledged for the currently available bone health assessment technologies. One example is the work of Degennaro et al.[17] in which a significant BMD reduction has been detected in pregnant women compared to non-pregnant women for the very first time. Several international working groups has used REMS technology for research purposes: Bojincă et al.[18] has proven the effectiveness of REMS BMD estimates in patients affected by rheumatoid arthritis. Kirilova et al.[19] assessed the values of lumbar spine and hip REMS-based BMD in premenopausal and postmenopausal women. In Khu et al.[20] REMS has been used for characterizing the relationship between body mass index and bone health. The growing interest towards REMS is also demonstrated by the publication of scientific review papers focused on this technology.[21][22]
References
- Di Paola, M.; Gatti, D.; Viapiana, O.; Cianferotti, L.; Cavalli, L.; Caffarelli, C.; Conversano, F.; Quarta, E.; Pisani, P.; Girasole, G.; Giusti, A. (February 2019). "Radiofrequency echographic multispectrometry compared with dual X-ray absorptiometry for osteoporosis diagnosis on lumbar spine and femoral neck". Osteoporosis International. 30 (2): 391–402. doi:10.1007/s00198-018-4686-3. hdl:2158/1141316. ISSN 0937-941X. PMID 30178159. S2CID 52146954.
- Adami, Giovanni; Arioli, Giovanni; Bianchi, Gerolamo; Brandi, Maria Luisa; Caffarelli, Carla; Casciaro, Sergio; Cavalli, Loredana; Cianferotti, Luisella; Conversano, Francesco; Giusti, Andrea; Gonnelli, Stefano (2019-06-01). "Fri0465 Prediction of Incident Fragility Fractures Through Radiofrequency Echographic Multi Spectrometry(rems)". Annals of the Rheumatic Diseases. 78 (Suppl 2): 928. doi:10.1136/annrheumdis-2019-eular.6716. ISSN 0003-4967. S2CID 196575682.
- Adami, Giovanni; Arioli, Giovanni; Bianchi, Gerolamo; Brandi, Maria Luisa; Caffarelli, Carla; Cianferotti, Luisella; Gatti, Davide; Girasole, Giuseppe; Gonnelli, Stefano; Manfredini, Monica; Muratore, Maurizio (May 2020). "Radiofrequency echographic multi spectrometry for the prediction of incident fragility fractures: A 5-year follow-up study". Bone. 134: 115297. doi:10.1016/j.bone.2020.115297. PMID 32092480. S2CID 211476843.
- Ammann, P.; Rizzoli, R. (March 2003). "Bone strength and its determinants". Osteoporosis International. 14 (S3): 13–18. doi:10.1007/s00198-002-1345-4. ISSN 0937-941X. PMID 12730800. S2CID 33253540.
- Conversano, Francesco; Franchini, Roberto; Greco, Antonio; Soloperto, Giulia; Chiriacò, Fernanda; Casciaro, Ernesto; Aventaggiato, Matteo; Renna, Maria Daniela; Pisani, Paola; Di Paola, Marco; Grimaldi, Antonella (January 2015). "A Novel Ultrasound Methodology for Estimating Spine Mineral Density". Ultrasound in Medicine & Biology. 41 (1): 281–300. doi:10.1016/j.ultrasmedbio.2014.08.017. ISSN 0301-5629. PMID 25438845.
- Casciaro, Sergio; Peccarisi, Marco; Pisani, Paola; Franchini, Roberto; Greco, Antonio; De Marco, Tommaso; Grimaldi, Antonella; Quarta, Laura; Quarta, Eugenio; Muratore, Maruizio; Conversano, Francesco (June 2016). "An Advanced Quantitative Echosound Methodology for Femoral Neck Densitometry". Ultrasound in Medicine & Biology. 42 (6): 1337–1356. doi:10.1016/j.ultrasmedbio.2016.01.024. PMID 27033331.
- Cavalli, Loredana; Lombardi, Fiorella Anna; Perrone, Daniele; Brandi, Maria Luisa (2019-06-01). "Sat0537 the Rems Technique Is Not Affected by Arthrosis Artifact, Which Can Hinder the Densitometric Recognition of Osteoporosis". Annals of the Rheumatic Diseases. 78 (Suppl 2): 1361. doi:10.1136/annrheumdis-2019-eular.8056. ISSN 0003-4967. S2CID 220021691.
- Tomai Pitinca, Maria Dea. "USE OF REMS TECHNOLOGY IN PATIENTS WITH SPINE ARTIFACTS: A NEW TECHNOLOGY TO ASSESS BONE HEALTH" (PDF). Osteoporosis International. 31 (Suppl 1).
- Tomai Pitinca, Maria Dea. "REMS TECHNOLOGY IN DAILY PRACTICE: CLINICAL CASES" (PDF). Osteoporosis International. 31 (Suppl 1).
- Crespo, Diana Ovejero; Nogues, Xavier; Diez-Perez, Adolfo (2019-06-01). "Sat0710-Hpr Radiofrequency Echographic Multi Spectrometry Osteoporosis Diagnosis on Femoral Neck: A Spanish Clinical Experience". Annals of the Rheumatic Diseases. 78 (Suppl 2): 1457. doi:10.1136/annrheumdis-2019-eular.6227. ISSN 0003-4967. S2CID 219272850.
- Diez-Perez, A.; Adachi, J. D.; Agnusdei, D.; Bilezikian, J. P.; Compston, J. E.; Cummings, S. R.; Eastell, R.; Eriksen, E. F.; Gonzalez-Macias, J.; Liberman, U. A.; Wahl, D. A. (2012-12-01). "Treatment failure in osteoporosis". Osteoporosis International. 23 (12): 2769–2774. doi:10.1007/s00198-012-2093-8. hdl:11343/219300. ISSN 1433-2965. PMID 22836278. S2CID 35603408.
- Pisani, P.; Muratore, M.; Conversano, F.; Casciaro, E.; Paola, M. Di; Forcignanò, R.; Ciccarese, M.; Surico, G.; Quarta, L.; Quarta, E.; Costanza, D. (2017-06-01). "FRI0568 Echosound approach for short-term follow-up of the denosumab effect on bmd recovery against aromatase inhibitor impact in breast cancer patients". Annals of the Rheumatic Diseases. 76 (Suppl 2): 704. doi:10.1136/annrheumdis-2017-eular.4060. ISSN 0003-4967. S2CID 58150992.
- Quarta, E.; Ciardo, D.; Ciccarese, M.; Conversano, F.; Paola, M. Di; Forcignanò, R.; Grimaldi, A.; Lombardi, F. A.; Muratore, M.; Pisani, P.; Casciaro, S. (2020-06-01). "Sat0461 Short-Term Monitoring of Denosumab Effect in Breast Cancer Patients Receiving Aromatase Inhibitors Using Rems Technology on Lumbar Spine". Annals of the Rheumatic Diseases. 79 (Suppl 1): 1187–1188. doi:10.1136/annrheumdis-2020-eular.3806. ISSN 0003-4967. S2CID 225844240.
- Diez-Perez, Adolfo; Brandi, Maria Luisa; Al-Daghri, Nasser (October 2019). "Radiofrequency echographic multi-spectrometry for the in-vivo assessment of bone strength: state of the art—outcomes of an expert consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO)". Aging Clinical and Experimental Research. 31 (10): 1375–1389. doi:10.1007/s40520-019-01294-4. PMC 6763416. PMID 31422565.
- Greco, Antonio; Pisani, Paola; Conversano, Francesco (April 2017). "Ultrasound Fragility Score: An innovative approach for the assessment of bone fragility". Measurement. 101: 236–242. Bibcode:2017Meas..101..236G. doi:10.1016/j.measurement.2016.01.033. Retrieved 2020-11-27.
- Pisani, Paola; Greco, Antonio; Conversano, Francesco (April 2017). "A quantitative ultrasound approach to estimate bone fragility: A first comparison with dual X-ray absorptiometry". Measurement. 101: 243–249. Bibcode:2017Meas..101..243P. doi:10.1016/j.measurement.2016.07.033. Retrieved 2020-11-27.
- Degennaro, V. A.; Cagninelli, G.; Lombardi, F. A. (2020). "VP34.12: First assessment of maternal status during pregnancy by means of radiofrequency echographic multi-spectrometry technology". Ultrasound in Obstetrics & Gynecology. 56 (S1): 199. doi:10.1002/uog.22845. S2CID 222411513. Retrieved 2020-11-27.
- Bojincă, Violeta‑Claudia; Popescu, Claudiu; Decianu, Raluca‑Daniela (2019-07-08). "A novel quantitative method for estimating bone mineral density using B‑mode ultrasound and radiofrequency signals‑a pilot study on patients with rheumatoid arthritis". Experimental and Therapeutic Medicine. 18 (3): 1661–1668. doi:10.3892/etm.2019.7746. PMC 6676208. PMID 31410123.
- Elena Kirilova; Nikola Kirilov; Stoyanka Vladeva. "Bone mineral density of lumbar spine and femoral neck assessed by novel echographic approach-Radiofrequency Echographic Multi Spectrometry (REMS)". Clinical Cases in Mineral & Bone Metabolism. 16 (1).
- Khu, Adrian; Sumardi, Michael (2020-03-30). "A REMS Scan-Based Report on Relation Between Body Mass Index and Osteoporosis in Urban Population of Medan at Royal Prima Hospital". Majalah Kedokteran Bandung. 52 (1): 22–27. doi:10.15395/mkb.v52n1.1827. S2CID 216424337. Retrieved 2020-11-27.
- Maria Dea Tomai Pitinca; Valentina Francolini; Stefano Gonnelli. "REMS technique: Future perspectives in an Academic Hospital". Clinical Cases in Mineral and Bone Metabolism. 15 (2).
- Iwaszkiewicz, Cezary; Leszczyński, Piotr (2019). "Bone densitometry by radiofrequency echographic multi-spectrometry (REMS) in the diagnosis of osteoporosis". Forum Reumatologiczne. 5 (2): 81–88. doi:10.5603/FR.2019.0011. S2CID 198301826. Retrieved 2020-11-27.