Basic reproduction number
In epidemiology, the basic reproduction number, or basic reproductive number (sometimes called basic reproduction ratio or basic reproductive rate), denoted (pronounced R nought or R zero),[1] of an infection is the expected number of cases directly generated by one case in a population where all individuals are susceptible to infection.[2] The definition assumes that no other individuals are infected or immunized (naturally or through vaccination). Some definitions, such as that of the Australian Department of Health, add the absence of "any deliberate intervention in disease transmission".[3] The basic reproduction number is not necessarily the same as the effective reproduction number (usually written [t for time], sometimes ),[4] which is the number of cases generated in the current state of a population, which does not have to be the uninfected state. is a dimensionless number (persons infected per person infecting) and not a time rate, which would have units of time−1,[5] or units of time like doubling time.[6]
is not a biological constant for a pathogen as it is also affected by other factors such as environmental conditions and the behaviour of the infected population. values are usually estimated from mathematical models, and the estimated values are dependent on the model used and values of other parameters. Thus values given in the literature only make sense in the given context and it is recommended not to use obsolete values or compare values based on different models.[7] does not by itself give an estimate of how fast an infection spreads in the population.
The most important uses of are determining if an emerging infectious disease can spread in a population and determining what proportion of the population should be immunized through vaccination to eradicate a disease. In commonly used infection models, when the infection will be able to start spreading in a population, but not if . Generally, the larger the value of , the harder it is to control the epidemic. For simple models, the proportion of the population that needs to be effectively immunized (meaning not susceptible to infection) to prevent sustained spread of the infection has to be larger than .[8] This is the so-called Herd immunity threshold or herd immunity level. Here, herd immunity means that the disease cannot spread in the population because each infected person, on average, can only transmit the infection to less than one other contact.[9] Conversely, the proportion of the population that remains susceptible to infection in the endemic equilibrium is . However, this threshold is based on simple models that assume a fully mixed population with no structured relations between the individuals. For example, if there is some correlation between people's immunization (e.g., vaccination) status, then the formula may underestimate the herd immunity threshold.[9]
The basic reproduction number is affected by several factors, including the duration of infectivity of affected people, the infectiousness of the microorganism, and the number of susceptible people in the population that the infected people contact.
History
The roots of the basic reproduction concept can be traced through the work of Ronald Ross, Alfred Lotka and others,[10] but its first modern application in epidemiology was by George Macdonald in 1952,[11] who constructed population models of the spread of malaria. In his work he called the quantity basic reproduction rate and denoted it by . "Rate" in this context means per person, which makes dimensionless as required. Because this can be misleading to anyone who understands "rate" only in the sense per unit of time, "number" or "ratio" is now preferred.
Definitions in specific cases
Contact rate and infectious period
Suppose that infectious individuals make an average of infection-producing contacts per unit time, with a mean infectious period of . Then the basic reproduction number is:
This simple formula suggests different ways of reducing and ultimately infection propagation. It is possible to decrease the number of infection-producing contacts per unit time by reducing the number of contacts per unit time (for example staying at home if the infection requires contact with others to propagate) or the proportion of contacts that produces infection (for example wearing some sort of protective equipment). Hence, it can also be written as[12]
where is the rate of contact between susceptible and infected individuals and is the transmissibility, i.e, the probability of infection given a contact. It is also possible to decrease the infectious period by finding and then isolating, treating or eliminating (as is often the case with animals) infectious individuals as soon as possible.
With varying latent periods
Latent period is the transition time between contagion event and disease manifestation. In cases of diseases with varying latent periods, the basic reproduction number can be calculated as the sum of the reproduction numbers for each transition time into the disease. An example of this is tuberculosis (TB). Blower and coauthors calculated from a simple model of TB the following reproduction number:[13]
In their model, it is assumed that the infected individuals can develop active TB by either direct progression (the disease develops immediately after infection) considered above as FAST tuberculosis or endogenous reactivation (the disease develops years after the infection) considered above as SLOW tuberculosis.[14]
Heterogeneous populations
In populations that are not homogeneous, the definition of is more subtle. The definition must account for the fact that a typical infected individual may not be an average individual. As an extreme example, consider a population in which a small portion of the individuals mix fully with one another while the remaining individuals are all isolated. A disease may be able to spread in the fully mixed portion even though a randomly selected individual would lead to fewer than one secondary case. This is because the typical infected individual is in the fully mixed portion and thus is able to successfully cause infections. In general, if the individuals infected early in an epidemic are on average either more likely or less likely to transmit the infection than individuals infected late in the epidemic, then the computation of must account for this difference. An appropriate definition for in this case is "the expected number of secondary cases produced, in a completely susceptible population, produced by a typical infected individual".[15]
The basic reproduction number can be computed as a ratio of known rates over time: if an infectious individual contacts other people per unit time, if all of those people are assumed to contract the disease, and if the disease has a mean infectious period of , then the basic reproduction number is just . Some diseases have multiple possible latency periods, in which case the reproduction number for the disease overall is the sum of the reproduction number for each transition time into the disease.
Epidemic Models on Networks
In reality, diseases spread over networks of contact between people. Such a network can be represented mathematically with a graph and is called the contact network.[16] Every node in a contact network is a representation of an individual and each link (edge) between a pair of nodes represents the contact between them. Links in the contact networks may be used to transmit the disease between the individuals and each disease has its own dynamics on top of its contact network. For example, individuals in a population can be assigned to compartments with labels – for example, S, I, or R, (Susceptible, Infectious, or Recovered) and they progress between compartments. The order of the labels usually shows the flow patterns between the compartments; for instance, SIR means each individual is originally susceptible then changes to infectious and finally gets recovered and remained recovered (immune) forever. On the other hand, public health may apply some interventions such as vaccination or contact tracing to reduce the spread of an epidemic disease. The combination of disease dynamics under the influence of interventions, if any, on a contact network may be modeled with another network, known as a transmission network. In a transmission network, all the links are responsible for transmitting the disease. If such a network is a locally tree-like network, meaning that any local neighborhood in such a network takes the form of a tree, then the basic reproduction can be written in terms of the average excess degree of the transmission network such that:
where is the mean-degree (average degree) of the network and is the second moment of the transmission network degree distribution. It is, however, not always straightforward to find the transmission network out of the contact network and the disease dynamics.[17] For example, if a contact network can be approximated with an Erdős–Rényi graph with a Poissonian degree distribution, and the disease spreading parameters are as defined in the example above, such that is the transmission rate per person and the disease has a mean infectious period of , then the basic reproduction number is [18][19] since for a Poisson distribution.
Next-generation method
One way to calculate is to average the expected number of new infections over all possible infected types. The next-generation method is a general method of deriving when more than one class of infectives is involved. This method, originally introduced by Diekmann et al. (1990),[20] can be used for models with underlying age structure or spatial structure, among other possibilities.[21] In this picture, the spectral radius of the next-generation matrix gives the basic reproduction number, [22]
Consider a sexually transmitted disease. In a naive population where almost everyone is susceptible, but the infection seed, if the expected number of gender 1 is and the expected number of infected gender 2 is , we can know how many would be infected in the next-generation. Such that the next-generation matrix can be written as:[12]
where each element is the expected number of secondary infections of gender caused by a single infected individual of gender , assuming that the population of gender is entirely susceptible. Diagonal elements are zero because people of the same gender cannot transmit the disease to each other but, for example, each can transmit the disease to , on average. Meaning that each element is a reproduction number, but one where who infects whom is accounted for. If generation is represented with then the next generation would be .
The spectral radius of the next-generation matrix is the basic reproduction number, , that is here, the geometric mean of the expected number of each gender in the next-generation. Note that multiplication factors and alternate because, the infectious person has to ‘pass through’ a second gender before it can enter a new host of the first gender. In other words, it takes two generations to get back to the same type, and every two generations numbers are multiplied by ×. The average per generation multiplication factor is therefore . Note that is a non-negative matrix so it has single, unique, positive, real eigenvalue which is strictly greater than all the others.
Next-generation matrix for compartmental models
In mathematical modelling of infectious disease, the dynamics of spreading is usually described through a set of non-linear ordinary differential equations (ODE). So there is always coupled equations of form which shows how the number of people in compartment changes over time. For example, in a SIR model, , , and . Compartmental models have a disease-free equilibrium (DFE) meaning that it is possible to find an equilibrium while setting the number of infected people to zero, . In other words, as a rule, there is an infection-free steady state. This solution, also usually ensures that the disease-free equilibrium is also an equilibrium of the system. There is another fixed point known as an Endemic Equilibrium (EE) where the disease is not totally eradicated and remains in the population. Mathematically, is a threshold for stability of a disease-free equilibrium such that:
To calculate , the first step is to linearise around the disease-free equilibrium (DFE), but for the infected subsystem of non-linear ODEs which describe the production of new infections and changes in state among infected individuals. Epidemiologically, the linearisation reflects that characterizes the potential for initial spread of an infectious person in a naive population, assuming the change in the susceptible population is negligible during the initial spread.[23] A linear system of ODEs can always be described by a matrix. So, the next step is to construct a linear positive operator that provides the next generation of infected people when applied to the present generation. Note that this operator (matrix) is responsible for the number of infected people, not all the compartments. Iteration of this operator describes the initial progression of infection within the heterogeneous population. So comparing the spectral radius of this operator to unity determines whether the generations of infected people grow or not. can be written as a product of the infection rate near the disease-free equilibrium and average duration of infectiousness. It is used to find the peak and final size of an epidemic.
The SEIR model with vital dynamics and constant population
As described in the example above, so many epidemic processes can be described with a SIR model. However, for many important infections, such as COVID-19, there is a significant latency period during which individuals have been infected but are not yet infectious themselves. During this period the individual is in compartment E (for exposed). Here, the formation of the next-generation matrix from the SEIR model involves determining two compartments, infected and non-infected, since they are the populations that spread the infection. So we only need to model the exposed, E, and infected, I, compartments. Consider a population characterized by a death rate and birth rate where a communicable disease is spreading. As in the previous example, we can use the transition rates between the compartments per capita such that be the infection rate, be the recovery rate, and be the rate at which a latent individual becomes infectious. Then, we can define the model dynamics using the following equations:[21][24]
Here we have 4 compartments and we can define vector where denotes the number or proportion of individuals in the -th compartment. Let be the rate of appearance of new infections in compartment such that it includes only infections that are newly arising, but does not include terms which describe the transfer of infectious individuals from one infected compartment to another. Then if is the rate of transfer of individuals into compartment by all other means and is the rate of transfer of individuals out of the -th compartment, then the difference gives the rate of change of such that .
We can now make matrices of partial derivatives of and such that
and , where is the disease-free equilibrium.
We now can form the next-generation matrix (operator) .[15][22] Basically, is a non-negative matrix which represents the infection rates near the equilibrium, and is an M-matrix for linear transition terms making a matrix which represents the average duration of infectiousness. Therefore, gives the rate at which infected individuals in produce new infections in , times the average length of time an individual spends in a single visit to compartment
Finally, for this SEIR process we can have:
and and so
Estimation methods
The basic reproduction number can be estimated through examining detailed transmission chains or through genomic sequencing. However, it is most frequently calculated using epidemiological models.[25] During an epidemic, typically the number of diagnosed infections over time is known. In the early stages of an epidemic, growth is exponential, with a logarithmic growth rate
For exponential growth, can be interpreted as the cumulative number of diagnoses (including individuals who have recovered) or the present number of infection cases; the logarithmic growth rate is the same for either definition. In order to estimate , assumptions are necessary about the time delay between infection and diagnosis and the time between infection and starting to be infectious.
In exponential growth, is related to the doubling time as
Simple model
If an individual, after getting infected, infects exactly new individuals only after exactly a time (the serial interval) has passed, then the number of infectious individuals over time grows as
or
The underlying matching differential equation is
or
In this case, or .
For example, with and , we would find .
If is time dependent
showing that it may be important to keep below 0, time-averaged, to avoid exponential growth.
Latent infectious period, isolation after diagnosis
In this model, an individual infection has the following stages:
- Exposed: an individual is infected, but has no symptoms and does not yet infect others. The average duration of the exposed state is .
- Latent infectious: an individual is infected, has no symptoms, but does infect others. The average duration of the latent infectious state is . The individual infects other individuals during this period.
- Isolation after diagnosis: measures are taken to prevent further infections, for example by isolating the infected person.
This is a SEIR model and may be written in the following form[26]
This estimation method has been applied to COVID-19 and SARS. It follows from the differential equation for the number of exposed individuals and the number of latent infectious individuals ,
The largest eigenvalue of the matrix is the logarithmic growth rate , which can be solved for .
In the special case , this model results in , which is different from the simple model above (). For example, with the same values and , we would find , rather than the true value of . The difference is due to a subtle difference in the underlying growth model; the matrix equation above assumes that newly infected patients are currently already contributing to infections, while in fact infections only occur due to the number infected at ago. A more correct treatment would require the use of delay differential equations.[27]
Effective reproduction number
In reality, varying proportions of the population are immune to any given disease at any given time. To account for this, the effective reproduction number or is used. is the average number of new infections caused by a single infected individual at time t in the partially susceptible population. It can be found by multiplying by the fraction S of the population that is susceptible. When the fraction of the population that is immune increases (i. e. the susceptible population S decreases) so much that drops below 1 in a basic SIR simulation, "herd immunity" has been achieved and the number of cases occurring in the population will gradually decrease to zero.[28][29][30]
Limitations of R0
Use of in the popular press has led to misunderstandings and distortions of its meaning. can be calculated from many different mathematical models. Each of these can give a different estimate of , which needs to be interpreted in the context of that model. Therefore, the contagiousness of different infectious agents cannot be compared without recalculating with invariant assumptions. values for past outbreaks might not be valid for current outbreaks of the same disease. Generally speaking, can be used as a threshold, even if calculated with different methods: if , the outbreak will die out, and if , the outbreak will expand. In some cases, for some models, values of can still lead to self-perpetuating outbreaks. This is particularly problematic if there are intermediate vectors between hosts (as is the case for zoonoses), such as malaria.[31] Therefore, comparisons between values from the "Values of of well-known infectious diseases" table should be conducted with caution.
Although cannot be modified through vaccination or other changes in population susceptibility, it can vary based on a number of biological, sociobehavioral, and environmental factors.[7] It can also be modified by physical distancing and other public policy or social interventions,[32][7] although some historical definitions exclude any deliberate intervention in reducing disease transmission, including nonpharmacological interventions.[3] And indeed, whether nonpharmacological interventions are included in often depends on the paper, disease, and what if any intervention is being studied.[7] This creates some confusion, because is not a constant; whereas most mathematical parameters with "nought" subscripts are constants.
depends on many factors, many of which need to be estimated. Each of these factors adds to uncertainty in estimates of . Many of these factors are not important for informing public policy. Therefore, public policy may be better served by metrics similar to , but which are more straightforward to estimate, such as doubling time or half-life ().[33][34]
Methods used to calculate include the survival function, rearranging the largest eigenvalue of the Jacobian matrix, the next-generation method,[35] calculations from the intrinsic growth rate,[36] existence of the endemic equilibrium, the number of susceptibles at the endemic equilibrium, the average age of infection[37] and the final size equation.[38] Few of these methods agree with one another, even when starting with the same system of differential equations.[31] Even fewer actually calculate the average number of secondary infections. Since is rarely observed in the field and is usually calculated via a mathematical model, this severely limits its usefulness.[39]
Sample values for various infectious diseases
Despite the difficulties in estimating mentioned in the previous section, estimates have been made for a number of genera, and are shown in this table. Each genus may be composed of many species, strains, or variants. Estimations of for species, strains, and variants are typically less accurate than for genera, and so are provided in separate tables below for diseases of particular interest (influenza and COVID-19).
| Disease | Transmission | R0 | HIT[lower-alpha 1] |
|---|---|---|---|
| Measles | Aerosol | 12–18[40][7] | 92–94% |
| Chickenpox (varicella) | Aerosol | 10–12[41] | 90–92% |
| Mumps | Respiratory droplets | 10–12[42] | 90–92% |
| Rubella | Respiratory droplets | 6–7[lower-alpha 2] | 83–86% |
| Polio | Fecal–oral route | 5–7[lower-alpha 2] | 80–86% |
| Pertussis | Respiratory droplets | 5.5[47] | 82% |
| Smallpox | Respiratory droplets | 3.5–6.0[48] | 71–83% |
| HIV/AIDS | Body fluids | 2–5[49] | 50–80% |
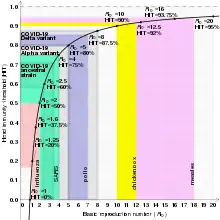
| COVID-19 (ancestral strain) | Respiratory droplets and aerosol[50] | 2.9 (2.4–3.4) | 65% (58–71%) |
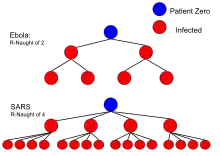
| SARS | Respiratory droplets | 2–4[52] | 50–75% |
| Diphtheria | Saliva | 2.6 (1.7–4.3)[53] | 62% (41–77%) |
| Common cold (e.g., rhinovirus) | Respiratory droplets | 2–3[54] | 50–67% |
| Mpox | Physical contact, body fluids, respiratory droplets | 2.1 (1.5–2.7)[55] | 53% (31–63%) |
| 2022–2023 mpox outbreak | Physical contact, body fluids, respiratory droplets, Sexual (MSM) | 1.2810 (1.0714–1.5508)[56] | 21.94% |
| Ebola (2014 outbreak) | Body fluids | 1.8 (1.4–1.8)[57] | 44% (31–44%) |
| Influenza (seasonal strains) | Respiratory droplets | 1.3 (1.2–1.4)[58] | 23% (17–29%) |
| Andes hantavirus | Respiratory droplets and body fluids | 1.2 (0.8–1.6)[59] | 16% (0–36%)[lower-alpha 3] |
| Nipah virus | Body fluids | 0.5[60] | 0%[lower-alpha 3] |
| MERS | Respiratory droplets | 0.5 (0.3–0.8)[61] | 0%[lower-alpha 3] |
Estimates for strains of influenza.
| Disease | Transmission | R0 | HIT[lower-alpha 1] |
|---|---|---|---|
| Influenza (1918 pandemic strain) | Respiratory droplets | 2[62] | 50% |
| Influenza (2009 pandemic strain) | Respiratory droplets | 1.6 (1.3–2.0)[2] | 37% (25–51%) |
| Influenza (seasonal strains) | Respiratory droplets | 1.3 (1.2–1.4)[58] | 23% (17–29%) |
Estimates for variants of SARS-CoV-2.
| Disease | Transmission | R0 | HIT[lower-alpha 1] |
|---|---|---|---|
| COVID-19 (Omicron variant) | Respiratory droplets and aerosol | 9.5[63] | 89% |
| COVID-19 (Delta variant) | Respiratory droplets and aerosol | 5.1[64] | 80% |
| COVID-19 (Alpha variant) | Respiratory droplets and aerosol | 4–5[65] | 75–80% |
| COVID-19 (ancestral strain) | Respiratory droplets and aerosol[50] | 2.9 (2.4–3.4) | 65% (58–71%) |
In popular culture
In the 2011 film Contagion, a fictional medical disaster thriller, a blogger's calculations for are presented to reflect the progression of a fatal viral infection from isolated cases to a pandemic.[32]
See also
Notes
- Compartmental models in epidemiology describe disease dynamics over time in a population of susceptible (S), infectious (I), and recovered (R) people using the SIR model. Note that in the SIR model, and are different quantities – the former describes the number of recovered at t = 0 whereas the latter describes the ratio between the frequency of contacts to the frequency of recovery.
- Held L, Hens N, O'Neill PD, Wallinga J (November 7, 2019). Handbook of Infectious Disease Data Analysis. CRC Press. p. 347. ISBN 978-1-351-83932-7. According to Guangdong Provincial Center for Disease Control and Prevention, "The effective reproductive number (R or Re is more commonly used to describe transmissibility, which is defined as the average number of secondary cases generated by per [sic] infectious case." For example, by one preliminary estimate during the ongoing pandemic, the effective reproductive number for SARS-CoV-2 was found to be 2.9, whereas for SARS it was 1.77.
References
- Milligan GN, Barrett AD (2015). Vaccinology : an essential guide. Chichester, West Sussex: Wiley Blackwell. p. 310. ISBN 978-1-118-63652-7. OCLC 881386962.
- Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, et al. (June 2009). "Pandemic potential of a strain of influenza A (H1N1): early findings". Science. 324 (5934): 1557–61. Bibcode:2009Sci...324.1557F. doi:10.1126/science.1176062. PMC 3735127. PMID 19433588.
- Becker NG, Glass K, Barnes B, Caley P, Philp D, McCaw JM, et al. (April 2006). "The reproduction number". Using Mathematical Models to Assess Responses to an Outbreak of an Emerged Viral Respiratory Disease. National Centre for Epidemiology and Population Health. ISBN 1-74186-357-0. Archived from the original on February 1, 2020. Retrieved February 1, 2020.
- Adam D (July 2020). "A guide to R - the pandemic's misunderstood metric". Nature. 583 (7816): 346–348. Bibcode:2020Natur.583..346A. doi:10.1038/d41586-020-02009-w. PMID 32620883.
- Jones J. "Notes On R0" (PDF). Stanford University.
- Siegel E. "Why 'Exponential Growth' Is So Scary For The COVID-19 Coronavirus". Forbes. Retrieved March 19, 2020.
- Delamater PL, Street EJ, Leslie TF, Yang YT, Jacobsen KH (January 2019). "Complexity of the Basic Reproduction Number (R0)". Emerging Infectious Diseases. 25 (1): 1–4. doi:10.3201/eid2501.171901. PMC 6302597. PMID 30560777.
- Fine, P.; Eames, K.; Heymann, D. L. (April 1, 2011). "'Herd Immunity': A Rough Guide". Clinical Infectious Diseases. 52 (7): 911–916. doi:10.1093/cid/cir007. PMID 21427399.
- Hiraoka, Takayuki; K. Rizi, Abbas; Kivelä, Mikko; Saramäki, Jari (May 12, 2022). "Herd immunity and epidemic size in networks with vaccination homophily". Physical Review E. 105 (5): L052301. arXiv:2112.07538. Bibcode:2022PhRvE.105e2301H. doi:10.1103/PhysRevE.105.L052301. PMID 35706197. S2CID 245130970.
- Smith DL, Battle KE, Hay SI, Barker CM, Scott TW, McKenzie FE (April 5, 2012). "Ross, macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens". PLOS Pathogens. 8 (4): e1002588. doi:10.1371/journal.ppat.1002588. PMC 3320609. PMID 22496640.
- Macdonald G (September 1952). "The analysis of equilibrium in malaria". Tropical Diseases Bulletin. 49 (9): 813–29. PMID 12995455.
- J.H. Jones, Notes on R0. Stanford University (2007).
- Blower SM, McLean AR, Porco TC, Small PM, Hopewell PC, Sanchez MA, Moss AR (August 1995). "The intrinsic transmission dynamics of tuberculosis epidemics". Nature Medicine. 1 (8): 815–21. doi:10.1038/nm0895-815. PMID 7585186. S2CID 19795498.
- Ma Y, Horsburgh CR, White LF, Jenkins HE (September 2018). "Quantifying TB transmission: a systematic review of reproduction number and serial interval estimates for tuberculosis". Epidemiology and Infection. 146 (12): 1478–1494. doi:10.1017/S0950268818001760. PMC 6092233. PMID 29970199.
- Diekmann O, Heesterbeek JA, Metz JA (1990). "On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations". Journal of Mathematical Biology. 28 (4): 365–82. doi:10.1007/BF00178324. hdl:1874/8051. PMID 2117040. S2CID 22275430.
- Network Science by Albert-László Barabási.
- Kenah, Eben; Robins, James M. (September 2007). "Second look at the spread of epidemics on networks". Physical Review E. 76 (3 Pt 2): 036113. arXiv:q-bio/0610057. Bibcode:2007PhRvE..76c6113K. doi:10.1103/PhysRevE.76.036113. ISSN 1539-3755. PMC 2215389. PMID 17930312.
- Pastor-Satorras, Romualdo; Castellano, Claudio; Van Mieghem, Piet; Vespignani, Alessandro (August 31, 2015). "Epidemic processes in complex networks". Reviews of Modern Physics. 87 (3): 925–979. arXiv:1408.2701. Bibcode:2015RvMP...87..925P. doi:10.1103/RevModPhys.87.925. S2CID 14306926.
- K. Rizi, Abbas; Faqeeh, Ali; Badie-Modiri, Arash; Kivelä, Mikko (April 20, 2022). "Epidemic spreading and digital contact tracing: Effects of heterogeneous mixing and quarantine failures". Physical Review E. 105 (4): 044313. arXiv:2103.12634. Bibcode:2022PhRvE.105d4313R. doi:10.1103/PhysRevE.105.044313. PMID 35590624. S2CID 232320251.
- Diekmann, O.; Heesterbeek, J. A. P.; Metz, J. A. J. (June 1, 1990). "On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations". Journal of Mathematical Biology. 28 (4): 365–382. doi:10.1007/BF00178324. hdl:1874/8051. ISSN 1432-1416. PMID 2117040. S2CID 22275430.
- Heffernan, J.M; Smith, R.J; Wahl, L.M (September 22, 2005). "Perspectives on the basic reproductive ratio". Journal of the Royal Society Interface. 2 (4): 281–293. doi:10.1098/rsif.2005.0042. ISSN 1742-5689. PMC 1578275. PMID 16849186.
- van den Driessche, P.; Watmough, James (November 1, 2002). "Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission". Mathematical Biosciences. 180 (1): 29–48. doi:10.1016/S0025-5564(02)00108-6. ISSN 0025-5564. PMID 12387915. S2CID 17313221.
- Diekmann, Odo; Heesterbeek, Hans; Britton, Tom (November 18, 2012). Mathematical Tools for Understanding Infectious Disease Dynamics. Princeton University Press. ISBN 978-0-691-15539-5.
- van den Driessche, P.; Watmough, James (2008), Brauer, Fred; van den Driessche, Pauline; Wu, Jianhong (eds.), "Further Notes on the Basic Reproduction Number", Mathematical Epidemiology, Lecture Notes in Mathematics, Berlin, Heidelberg: Springer, vol. 1945, pp. 159–178, doi:10.1007/978-3-540-78911-6_6, ISBN 978-3-540-78911-6, retrieved November 8, 2022
- Wohl S, Schaffner SF, Sabeti PC (September 2016). "Genomic Analysis of Viral Outbreaks". Annual Review of Virology. 3 (1): 173–195. doi:10.1146/annurev-virology-110615-035747. PMC 5210220. PMID 27501264.
- Lipsitch M, Cohen T, Cooper B, Robins JM, Ma S, James L, et al. (June 2003). "Transmission dynamics and control of severe acute respiratory syndrome". Science. 300 (5627): 1966–70. Bibcode:2003Sci...300.1966L. doi:10.1126/science.1086616. PMC 2760158. PMID 12766207.
- Rihan, Fathalla A.; Anwar, M. Naim (2012). "Qualitative Analysis of Delayed SIR Epidemic Model with a Saturated Incidence Rate". International Journal of Differential Equations. 2012: 1–13. doi:10.1155/2012/408637.
- Garnett GP (February 2005). "Role of herd immunity in determining the effect of vaccines against sexually transmitted disease". The Journal of Infectious Diseases. 191 (Suppl 1): S97-106. doi:10.1086/425271. PMID 15627236.
- Rodpothong P, Auewarakul P (October 2012). "Viral evolution and transmission effectiveness". World Journal of Virology. 1 (5): 131–4. doi:10.5501/wjv.v1.i5.131. PMC 3782273. PMID 24175217.
- Dabbaghian V, Mago VK (2013). Theories and Simulations of Complex Social Systems. Springer. pp. 134–35. ISBN 978-3642391491. Retrieved March 29, 2015.
- Li J, Blakeley D, Smith RJ (2011). "The failure of R0". Computational and Mathematical Methods in Medicine. 2011 (527610): 527610. doi:10.1155/2011/527610. PMC 3157160. PMID 21860658.
- Byrne M (October 6, 2014), "The Misunderstood Number That Predicts Epidemics", vice.com, retrieved March 23, 2020
- Balkew TM (December 2010). The SIR Model When S(t) is a Multi-Exponential Function (Thesis). East Tennessee State University.
- Ireland MW, ed. (1928). The Medical Department of the United States Army in the World War, vol. IX: Communicable and Other Diseases. Washington: U.S.: U.S. Government Printing Office. pp. 116–7.
- Diekmann O, Heesterbeek JA (2000). "The Basic Reproduction Ratio". Mathematical Epidemiology of Infectious Diseases : Model Building, Analysis and Interpretation. New York: Wiley. pp. 73–98. ISBN 0-471-49241-8.
- Chowell G, Hengartner NW, Castillo-Chavez C, Fenimore PW, Hyman JM (July 2004). "The basic reproductive number of Ebola and the effects of public health measures: the cases of Congo and Uganda". Journal of Theoretical Biology. 229 (1): 119–26. arXiv:q-bio/0503006. Bibcode:2004JThBi.229..119C. doi:10.1016/j.jtbi.2004.03.006. PMID 15178190. S2CID 7298792.
- Ajelli M, Iannelli M, Manfredi P, Ciofi degli Atti ML (March 2008). "Basic mathematical models for the temporal dynamics of HAV in medium-endemicity Italian areas". Vaccine. 26 (13): 1697–707. doi:10.1016/j.vaccine.2007.12.058. PMID 18314231.
- von Csefalvay, Chris (January 1, 2023), von Csefalvay, Chris (ed.), "2 - Simple compartmental models: The bedrock of mathematical epidemiology", Computational Modeling of Infectious Disease, Academic Press, pp. 19–91, doi:10.1016/b978-0-32-395389-4.00011-6, ISBN 978-0-323-95389-4, retrieved March 2, 2023
- Heffernan JM, Smith RJ, Wahl LM (September 2005). "Perspectives on the basic reproductive ratio". Journal of the Royal Society, Interface. 2 (4): 281–93. doi:10.1098/rsif.2005.0042. PMC 1578275. PMID 16849186.
- Guerra FM, Bolotin S, Lim G, Heffernan J, Deeks SL, Li Y, Crowcroft NS (December 2017). "The basic reproduction number (R0) of measles: a systematic review". The Lancet. Infectious Diseases. 17 (12): e420–e428. doi:10.1016/S1473-3099(17)30307-9. PMID 28757186.
- Ireland's Health Services. Health Care Worker Information (PDF). Retrieved March 27, 2020.
- Australian government Department of Health Mumps Laboratory Case Definition (LCD)
- Centers for Disease Control and Prevention; World Health Organization (2001). "History and epidemiology of global smallpox eradication". Smallpox: disease, prevention, and intervention (training course) (Presentation). Atlanta: Centers for Disease Control and Prevention (published August 25, 2014). cdc:27929. Archived (PDF) from the original on March 17, 2017. Retrieved June 17, 2021.
- Fine, Paul E. M. (1993). "Herd Immunity: History, Theory, Practice". Epidemiologic Reviews. 15 (2): 265–302. doi:10.1093/oxfordjournals.epirev.a036121. PMID 8174658.
- Luman, ET; Barker, LE; Simpson, DM; Rodewald, LE; Szilagyi, PG; Zhao, Z (May 2001). "National, state, and urban-area vaccination-coverage levels among children aged 19–35 months, United States, 1999". American Journal of Preventive Medicine. 20 (4): 88–153. doi:10.1016/s0749-3797(01)00274-4. PMID 12174806.
- Jiles, RB; Fuchs, C; Klevens, RM (September 22, 2000). "Vaccination coverage among children enrolled in Head Start programs or day care facilities or entering school". Morbidity and Mortality Weekly Report. 49 (9): 27–38. PMID 11016876.
- Kretzschmar M, Teunis PF, Pebody RG (June 2010). "Incidence and reproduction numbers of pertussis: estimates from serological and social contact data in five European countries". PLOS Medicine. 7 (6): e1000291. doi:10.1371/journal.pmed.1000291. PMC 2889930. PMID 20585374.
- Gani R, Leach S (December 2001). "Transmission potential of smallpox in contemporary populations". Nature. 414 (6865): 748–51. Bibcode:2001Natur.414..748G. doi:10.1038/414748a. PMID 11742399. S2CID 52799168. Retrieved March 18, 2020.
- "Playing the Numbers Game: R0". National Emerging Special Pathogen Training and Education Center. January 30, 2020. Archived from the original on May 12, 2020. Retrieved December 27, 2020.
[...] while infections that require sexual contact like HIV have a lower R0 (2-5).
- Prather, Kimberly A.; Marr, Linsey C.; Schooley, Robert T.; McDiarmid, Melissa A.; Wilson, Mary E.; Milton, Donald K. (October 16, 2020). "Airborne transmission of SARS-CoV-2". Science. 370 (6514): 303.2–304. Bibcode:2020Sci...370..303P. doi:10.1126/science.abf0521. PMID 33020250. S2CID 222145689.
- Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). Department of Communicable Disease Surveillance and Response (Technical report). World Health Organization. p. 26. hdl:10665/70863. WHO/CDS/CSR/GAR/2003.11.
A number of researchers have estimated the basic reproduction number by fitting models to the initial growth of epidemics in a number of countries. Their observations indicate that the SARS-CoV is less transmissible than initially thought with estimates of Ro in the range of 2-4.
- Truelove SA, Keegan LT, Moss WJ, Chaisson LH, Macher E, Azman AS, Lessler J (June 2020). "Clinical and Epidemiological Aspects of Diphtheria: A Systematic Review and Pooled Analysis". Clinical Infectious Diseases. 71 (1): 89–97. doi:10.1093/cid/ciz808. PMC 7312233. PMID 31425581.
- Freeman C (November 6, 2014). "Magic formula that will determine whether Ebola is beaten". The Telegraph. Telegraph.Co.Uk. Archived from the original on January 12, 2022. Retrieved March 30, 2020.
- Grant R, Nguyen LL, Breban R (September 1, 2020). "Modelling human-to-human transmission of monkeypox" (PDF). Bulletin of the World Health Organization. 98 (9): 638–640. doi:10.2471/BLT.19.242347. ISSN 0042-9686. PMC 7463189. PMID 33012864. Archived from the original (PDF) on December 11, 2020.
- Al-Raeei M (February 2023). "The study of human monkeypox disease in 2022 using the epidemic models: herd immunity and the basic reproduction number case". Annals of Medicine & Surgery. 85 (2): 316–321. doi:10.1097/MS9.0000000000000229. ISSN 2049-0801. PMC 9949786. PMID 36845803.
- Wong ZS, Bui CM, Chughtai AA, Macintyre CR (April 2017). "A systematic review of early modelling studies of Ebola virus disease in West Africa". Epidemiology and Infection. 145 (6): 1069–1094. doi:10.1017/S0950268817000164. PMC 9507849. PMID 28166851.
The median of the R0 mean estimate for the ongoing epidemic (overall) is 1.78 (interquartile range: 1.44, 1.80)
- Chowell G, Miller MA, Viboud C (June 2008). "Seasonal influenza in the United States, France, and Australia: transmission and prospects for control". Epidemiology and Infection. Cambridge University Press. 136 (6): 852–64. doi:10.1017/S0950268807009144. PMC 2680121. PMID 17634159.
The reproduction number across influenza seasons and countries lied in the range 0.9–2.0 with an overall mean of 1.3, and 95% confidence interval (CI) 1.2–1.4.
- Martínez, Valeria P.; Di Paola, Nicholas; Alonso, Daniel O.; Pérez-Sautu, Unai; Bellomo, Carla M.; Iglesias, Ayelén A.; et al. (December 3, 2020). "'Super-Spreaders' and Person-to-Person Transmission of Andes Virus in Argentina". New England Journal of Medicine. 383 (23): 2230–2241. doi:10.1056/NEJMoa2009040. PMID 33264545. S2CID 227259435.
- Luby SP (October 2013). "The pandemic potential of Nipah virus". Antiviral Research. 100 (1): 38–43. doi:10.1016/j.antiviral.2013.07.011. PMID 23911335.
- Kucharski AJ, Althaus CL (June 2015). "The role of superspreading in Middle East respiratory syndrome coronavirus (MERS-CoV) transmission". Euro Surveillance. 20 (25): 14–8. doi:10.2807/1560-7917.ES2015.20.25.21167. PMID 26132768.
- "Omicron transmission: how contagious diseases spread". Nebraska Medicine. December 21, 2021. Retrieved January 25, 2022.
- Liu, Y (March 9, 2022). "The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta". Journal of Travel Medicine. 29 (3). Table 1. doi:10.1093/jtm/taac037. ISSN 1708-8305. PMC 8992231. PMID 35262737.
- Liu, Ying; Rocklöv, Joacim (October 1, 2021). "The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus". Journal of Travel Medicine. 28 (7). doi:10.1093/jtm/taab124. ISSN 1708-8305. PMC 8436367. PMID 34369565.
- Gallagher, James (June 12, 2021). "Covid: Is there a limit to how much worse variants can get?". BBC News. Retrieved July 21, 2021.
Further reading
- Heesterbeek, J.A.P. (2002). "A brief history of R0 and a recipe for its calculation". Acta Biotheoretica. 50 (3): 189–204. doi:10.1023/a:1016599411804. hdl:1874/383700. PMID 12211331. S2CID 10178944.
- Heffernan, J.M; Smith, R.J; Wahl, L.M (September 22, 2005). "Perspectives on the basic reproductive ratio". Journal of the Royal Society Interface. 2 (4): 281–293. doi:10.1098/rsif.2005.0042. PMC 1578275. PMID 16849186.
- Jones JH (May 1, 2007). "Notes on " (PDF). Retrieved November 6, 2018.
- Van Den Driessche, P.; Watmough, James (2008). "Further Notes on the Basic Reproduction Number". Mathematical Epidemiology. Lecture Notes in Mathematics. Vol. 1945. pp. 159–178. doi:10.1007/978-3-540-78911-6_6. ISBN 978-3-540-78910-9.