Reproductive toxicity
Reproductive toxicity refers to the potential risk from a given chemical, physical or biologic agent to adversely affect both male and female fertility as well as offspring development.[1] Reproductive toxicants may adversely affect sexual function, ovarian failure, fertility as well as causing developmental toxicity in the offspring.[2][3] Lowered effective fertility related to reproductive toxicity relates to both male and female effects alike and is reflected in decreased sperm counts, semen quality and ovarian failure. Infertility is medically defined as a failure of a couple to conceive over the course of one year of unprotected intercourse.[4] As many as 20% of couples experience infertility.[4] Among men, oligospermia is defined as a paucity of viable spermatozoa in the semen, whereas azoospermia refers to the complete absence of viable spermatozoa in the semen.[4]

The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) separates reproductive toxicity from germ cell mutagenicity and carcinogenicity, even though both these hazards may also affect fertility.
Effects
Many drugs can affect the human reproductive system. Their effects can be
- desired (hormonal contraception),
- a minor unwanted side effect (many antidepressants) or
- a major public health problem (thalidomide).
However, most studies of reproductive toxicity have focused on occupational or environmental exposure to chemicals and their effects on reproduction. Both consumption of alcohol and tobacco smoking are known to be "toxic for reproduction" in the sense used here.
One well-known group of substances which are toxic for reproduction are teratogens – substances which cause birth defects. (S)-thalidomide is possibly the most notorious of these.[5]
Another group of substances which have received much attention (and prompted some controversy) as possibly toxic for reproduction are the so-called endocrine disruptors.[5] Endocrine disruptors change how hormones are produced and how they interact with their receptors. Endocrine disruptors are classified as estrogenic, anti-estrogenic, androgenic or anti-androgenic. Each category includes pharmaceutical compounds and environmental compounds. Estrogenic or androgenic compounds will cause the same hormonal responses as the sex steroids (estrogen and testosterone). However anti-estrogenic and anti-andogenic compounds bind to a receptor and block the hormones from binding to their receptors, thus preventing their function. A few examples of the many types of endocrine disruptors are trenbolone (androgenic), flutamide (anti-androgenic), diethylstilbestrol (estrogenic), bisphenol A (estrogenic) and tributyltin (anti-estrogenic).[6][7]
However, many substances which are toxic for reproduction do not fall into any of these groups: lead compounds, for example, are considered to be toxic for reproduction[6][7] given their adverse effects on the normal intellectual and psychomotor development of human babies and children.
Examples
Lead

Lead, a heavy metal that can exist in both organic and inorganic forms, is associated with adverse effects on male libido, erectile disfunction, premature ejaculation and poor sperm quality.[8] Lead is believed to predominantly affect male reproduction by the disruption of hormones, which reduces the quantity of sperm production in the seminiferous tubules. It has also been proposed that lead causes poor semen quality by increasing reactive oxygen species due to lipid per-oxidation, leading to cellular damage.[9][10]
Cadmium

Cadmium is a heavy metal used in jewelry making, electronics, welding and galvanizing steel.[11] The human route of exposure is primarily inhalational or oral; environmental exposure among the non-occupationally exposed can occur due to exposure to cigarette smoking.[11] The oral route of exposure can occur due to ingesting plants and shellfish that have taken up cadmium from water and soil.[11] Exposure to cadmium results in adverse male fertility in terms of decreased spermatogenesis, semen quality, sperm motility and impaired hormonal synthesis.[12] Likewise, exposure to cadmium impairs female fertility in terms of menstrual cycle regularity and reproductive hormonal balance.[12]
Chromium
Hexavalent chromium ( Cr VI) is used in the electronics industry and for metal plating.[13] Chromium exposure is primarily inhalation or through ingestion.[14] Human and animal studies show that exposure to hexavalent chromium decreases semen quality and sperm counts.[15]
Mercury
Elemental mercury( Hg0) is a metal that exists as liquid form at room temperature and is commonly found in thermometers, blood pressure cuffs and dental amalgams. In terms of exposure, the route of absorption is primarily via inhalation through mercury vapor.[16] Data among female dental technicians exposed to mercury vapors have demonstrated decreased fertility among those who were exposed and practiced poor industrial hygiene while handling dental amalgams.[16][17] Among female workers in mercury smelting plants an increase in spontaneous abortions has been reported.[17]
.JPG.webp)
Dibromochloropropane
Dibromochloropropane (DBCP) is used as a pesticide against nematodes in the agricultural industry.[18] DBCP is one of the most well-known reproductive toxicants known to cause testicular toxicity.[8] Workers in chemical factories exposed to dibromochloropropane have been shown to develop dose-dependent oligospermia and azoospermia.[8] Additional studies also demonstrated that DBCP-exposed workers in banana and pineapple plantations in central America and other countries also developed oligospermia and azoospermia.[19] In 1977, the United States Environmental Protection Agency banned the use of DBCP in agriculture due to its effect on male fertility.[20] Despite being banned from use in agriculture, DBCP is still used as an intermediate in chemical manufacturing as well as a reagent in research.[20]
Ethylene dibromide
Ethylene dibromide (EDB) is a fumigant that was originally used to protect citrus fruits, grains and vegetables from insects.[21] Use of EDB in the United States was banned by the United States Environmental Protection Agency in 1984, however EDB is still used in the United States as fumigant to treat timber logs for beetles and termites.[21] Likewise, it is still used as an intermediate in chemical manufacturing.[21] Exposure to EDB has been shown to adversely affect male fertility by leading to a decreased sperm counts, decreased numbers of viable sperm and increased abnormal sperm morphology.[22][23] The primary route of exposure is through inhalation.[21]
Industrial solvents
Solvent exposure is common among men and women working in industrial settings. Specific solvents including xylene, perchloroethylene, toluene and methylene chloride have been shown to be associated with a concurrent elevation in risk for spontaneous abortion [24]
Ionizing radiation
Ionizing radiation in the form alpha, beta and gamma emissions are well known to adversely affect male fertility.[25] Exposure in the range of 0.1 to1.2 Gy is associated with spermatogonial injury; whereas between 4-6 Gy reductions of sperm counts have been reported.[25]
Radio frequency electromagnetic fields
Radio frequency electromagnetic fields, such as those generated from mobile phone devices, have been shown to decrease semen quality production in experimental animal models; however human data is still equivocal at best.[26][27] The International Association for the Research of Cancer(IARC) classifies radio frequency electromagnetic fields as a group 2B or possibly carcinogenic.[28]
Thalidomide
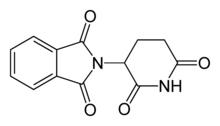
Thalidomide was once prescribed therapeutically from the 1950s to early 1960s in Europe as an anti-nausea medication to alleviate morning sickness among pregnant women. While the exact mechanism of action of thalidomide is not known, it is thought to be related to inhibition of angiogenesis through interaction with the insulin like growth factor(IGF-1) and fibroblast like growth factor 2 (FGF-2) pathways.[29] In the 1960s, it became apparent that thalidomide altered embryo development and led to limb deformities such as thumb absence, underdevelopment of entire limbs, or phocomelia.[29] Thalidomide may have caused teratogenic effects in over 10,000 babies worldwide.[30][31]
Endocrine disrupting compounds
Lipid soluble compounds that can cross the cell lipid bilayer and bind cytoplasmic steroid hormone receptors can translocate to the nucleus and act as estrogen agonists.[32] Diethylstilbestrol (DES), a synthetic estrogen, is one such endocrine disruptor and acts as an estrogen agonist. Diethylstilbestrol was used from 1938 to 1971 to prevent spontaneous abortions.[32] Diethylstilbestrol causes cancer and mutations by producing highly reactive metabolites, also causing DNA adducts to form. Exposure to diethylstilbestrol in the womb can cause atypical reproductive tract formation. Specifically, females exposed to diethylstilbestrol in utero during the first trimester have are more likely to develop clear cell vaginal carcinoma, and males have an increased risk of hypospadias.[33]
Bisphenol A
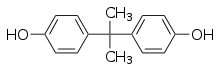
Bisphenol A (BPA) is used in polycarbonate plastic consumer goods and aluminum can liners.[34] BPA is an example of an endocrine disruptor which negatively affects reproductive development by acting as an estrogen mimicker (xenoestrogen) and a likely androgen mimicker.[35] Bisphenol A exposure in fetal female rats leads to mammary gland morphogenesis, increased formation of ovarian tumors, and increased risk of developing mammary gland neoplasia in adult life. In lab animal models, BPA is considered to be both an ovarian and uterine toxicant as it impairs endometrial proliferation, decreases uterine receptivity and decreases the chances for successful implantation of the embryo [36] The adverse reproductive toxicological impacts of bisphenol A have been better studied in females than in males.[37][38][36]
References
- Occupational Health and Safety Administration. "Reproductive Hazards". osha.gov. Retrieved 6 February 2022.
- "Regulation (EC) No 1272/2008 of the EUROPEAN PARLIAMENT and of the COUNCIL". Official Journal of the European Union. 16 December 2008.
Annex I, section 3.7 labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006.
- International Programme on Chemical Safety (2001). "Principles For Evaluating Health Risks To Reproduction Associated With Exposure To Chemicals". Environmental Health Criteria. Geneva: World Health Organization. 225.
- Long J (2022). Current Medical Diagnosis & Treatment. McGraw Hill.
- International Programme on Chemical Safety (2002). "Global assessment of the state-of-the-science of endocrine disruptors". Geneva: World Health Organization. WHO/PCS/EDC/02.2. Archived from the original on July 9, 2004.
- Commission Directive 2004/73/EC of 29 August 2004 adapting to technical progress for the 29th time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances. OJEC L152, 30.04.2004, pp. 1–311 (index no. 082-001-00-6).
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEC L353, 31.12.2008, pp. 1–1355 at p. 444 (index no. 082-001-00-6).
- LaDou J (2014). Current Diagnosis and Treatment: Occupational Mediccine. United States: McGraw Hill Medical. pp. 455–459. ISBN 978-1-25-925145-0.
- Vigeh M, Smith DR, Hsu PC (2011). "How does lead induce male infertility?". Iranian Journal of Reproductive Medicine. 9 (1): 1–8. PMC 4212138. PMID 25356074.
- Dorostghoal M, Seyyednejad SM, Jabari A (May 2014). "Protective effects of Fumaria parviflora L. on lead-induced testicular toxicity in male rats". Andrologia. 46 (4): 437–46. doi:10.1111/and.12100. PMID 23611729. S2CID 24028551.
- Bernhoft RA (2013). "Cadmium toxicity and treatment". TheScientificWorldJournal. 2013: 394652. doi:10.1155/2013/394652. PMC 3686085. PMID 23844395.
- Kumar S, Sharma A (December 2019). "Cadmium toxicity: effects on human reproduction and fertility". Reviews on Environmental Health. 34 (4): 327–338. doi:10.1515/reveh-2019-0016. PMID 31129655. S2CID 167205593.
- Pereira SC, Oliveira PF, Oliveira SR, Pereira ML, Alves MG (August 2021). "Impact of Environmental and Lifestyle Use of Chromium on Male Fertility: Focus on Antioxidant Activity and Oxidative Stress". Antioxidants. 10 (9): 1365. doi:10.3390/antiox10091365. PMC 8468676. PMID 34572997.
- Costa M, Klein CB (February 2006). "Toxicity and carcinogenicity of chromium compounds in humans". Critical Reviews in Toxicology. 36 (2): 155–163. doi:10.1080/10408440500534032. PMID 16736941. S2CID 85928480.
- Li H, Chen Q, Li S, Yao W, Li L, Shi X, et al. (October 2001). "Effect of Cr(VI) exposure on sperm quality: human and animal studies". The Annals of Occupational Hygiene. 45 (7): 505–511. doi:10.1016/S0003-4878(01)00004-7. PMID 11583652.
- Davis BJ, Price HC, O'Connor RW, Fernando R, Rowland AS, Morgan DL (February 2001). "Mercury vapor and female reproductive toxicity". Toxicological Sciences. 59 (2): 291–296. doi:10.1093/toxsci/59.2.291. PMID 11158722.
- Schuurs AH (May 1999). "Reproductive toxicity of occupational mercury. A review of the literature". Journal of Dentistry. 27 (4): 249–256. doi:10.1016/S0300-5712(97)00039-0. PMID 10193101.
- Babich H, Davis DL, Stotzky G (March 1981). "Dibromochloropropane (DBCP): a review". The Science of the Total Environment. 17 (3): 207–221. Bibcode:1981ScTEn..17..207B. doi:10.1016/0048-9697(81)90062-0. PMID 7015501.
- Marinaro J. "Environmental Toxins and Men's Health: Dibromochloropropane". Effects of Lifestyle on Men's Health – via Science Direct.
- Naistat DM (2014). Encyclopedia of Toxicology (Third ed.). Science Direct.
- "Ethylene Dibromide (Dibromoethane) Hazard Summary" (PDF). EPA.gov. Retrieved March 31, 2022.
- Schrader, Steven M. (2003). "Man and the workplace". Chemical Health & Safety. 10 (5): 11–16. doi:10.1016/s1074-9098(03)00089-3.
- Ratcliffe, J. M.; Schrader, S. M.; Steenland, K.; Clapp, D. E.; Turner, T.; Hornung, R. W. (1 May 1987). "Semen quality in papaya workers with long term exposure to ethylene dibromide". Occupational and Environmental Medicine. 44 (5): 317–326. doi:10.1136/oem.44.5.317. PMC 1007829. PMID 3297130. ProQuest 1771266376.
- Janssen S (2021). CURRENT Diagnosis & Treatment: Occupational & Environmental Medicine (6th ed.). McGraw Hill.
- Kesari KK, Agarwal A, Henkel R (December 2018). "Radiations and male fertility". Reproductive Biology and Endocrinology. 16 (1): 118. doi:10.1186/s12958-018-0431-1. PMC 6240172. PMID 30445985.
- Kesari KK, Kumar S, Nirala J, Siddiqui MH, Behari J (March 2013). "Biophysical evaluation of radiofrequency electromagnetic field effects on male reproductive pattern". Cell Biochemistry and Biophysics. 65 (2): 85–96. doi:10.1007/s12013-012-9414-6. PMID 22926544. S2CID 1710686.
- Liu K, Li Y, Zhang G, Liu J, Cao J, Ao L, Zhang S (July 2014). "Association between mobile phone use and semen quality: a systemic review and meta-analysis". Andrology. 2 (4): 491–501. doi:10.1111/j.2047-2927.2014.00205.x. PMID 24700791. S2CID 8711367.
- "Iarc Classifies Radiofrequency Electromagnetic Fields as Possibly Carcinogenic to Humans" (PDF). International Agency for the Research in Cancer, World Health Organization. 31 May 2011.
- Stephens TD, Bunde CJ, Fillmore BJ (June 2000). "Mechanism of action in thalidomide teratogenesis". Biochemical Pharmacology. 59 (12): 1489–1499. doi:10.1016/S0006-2952(99)00388-3. PMID 10799645.
- Kim JH, Scialli AR (July 2011). "Thalidomide: the tragedy of birth defects and the effective treatment of disease". Toxicological Sciences. 122 (1): 1–6. doi:10.1093/toxsci/kfr088. PMID 21507989.
- Martínez-Frías ML (June 2012). "[The thalidomide experience: review of its effects 50 years later]". Medicina Clinica (in Spanish). 139 (1): 25–32. doi:10.1016/j.medcli.2011.10.011. PMID 22177324.
- Sharara FI, Seifer DB, Flaws JA (October 1998). "Environmental toxicants and female reproduction". Fertility and Sterility. 70 (4): 613–22. doi:10.1016/s0015-0282(98)00253-2. PMID 9797086.
- Klip H, Verloop J, van Gool JD, Koster ME, Burger CW, van Leeuwen FE (March 2002). "Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study". Lancet. London, England. 359 (9312): 1102–7. doi:10.1016/S0140-6736(02)08152-7. PMID 11943257. S2CID 45769124.
- Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, et al. (August 2014). "Bisphenol a and reproductive health: update of experimental and human evidence, 2007-2013". Environmental Health Perspectives. 122 (8): 775–786. doi:10.1289/ehp.1307728. PMC 4123031. PMID 24896072.
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. (2007). "In vivo effects of bisphenol A in laboratory rodent studies". Reproductive Toxicology. 24 (2): 199–224. doi:10.1016/j.reprotox.2007.06.004. PMC 2151845. PMID 17683900.
- Rochester JR (December 2013). "Bisphenol A and human health: a review of the literature". Reproductive Toxicology. 42: 132–155. doi:10.1016/j.reprotox.2013.08.008. PMID 23994667.
- Jones BA, Wagner LS, Watson NV (August 2016). "The Effects of Bisphenol A Exposure at Different Developmental Time Points in an Androgen-Sensitive Neuromuscular System in Male Rats". Endocrinology. 157 (8): 2972–2977. doi:10.1210/en.2015-1574. PMID 27022676.
- Soto AM, Sonnenschein C (July 2010). "Environmental causes of cancer: endocrine disruptors as carcinogens". Nature Reviews. Endocrinology. 6 (7): 363–370. doi:10.1038/nrendo.2010.87. PMC 3933258. PMID 20498677.
Further reading
- Scott AJ, LaDou J (2001-04-16). "Biological Rhythms, Shiftwork, and Occupational Health". Patty's Toxicology. Hoboken, NJ, USA: John Wiley & Sons, Inc. doi:10.1002/0471435139.tox107. ISBN 978-0-471-12547-1.