Rimeporide
Rimeporide is an experimental drug for the treatment of Duchenne muscular dystrophy, being developed by the EspeRare foundation.[1] it has been granted orphan drug status by the European Medicines Agency.[2]
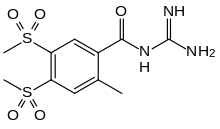 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H15N3O5S2 |
| Molar mass | 333.38 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mechanism of action
The substance blocks an ion pump called sodium–hydrogen antiporter 1. While the exact mechanism is unknown, it is speculated that inhibition of this pump reduces pH, sodium and calcium overload in cells of patients with Duchenne muscular dystrophy.[1]
History
Rimeporide was designed as a treatment for chronic heart failure. It was tested in seven Phase I studies clinical trials in patients with congestive heart failure and some degree of renal insufficiency. Subsequently, the drug was licensed to EspeRare, a Swiss nonprofit organisation[3] that aims at repositioning drugs for rare diseases. As of May 2015, the substance has demonstrated efficacy in several animal models of Duchenne muscular dystrophy.[4]
It has also been recently tested in young boys with Duchenne muscular Dystrophy aged 6 to 11 years.[5]
See also
Other drugs for Duchenne muscular dystrophy
- Ataluren
- Biostrophin (experimental)
- Idebenone (experimental)
References
- Spreitzer H (26 May 2015). "Neue Wirkstoffe – Rimeporid". Österreichische Apothekerzeitung (in German). 69 (11): 12.
- "EspeRare's Rimeporide receives Orphan Drug Designation in Duchenne Muscular Dystrophy". EspeRare. 4 May 2015.
- "Our mission and vision". EspeRare. Retrieved 23 July 2015.
- Ghaleh B, Barthélemy I, Wojcik J, Sambin L, Bizé A, Hittinger L, Tran TD, Thomé FP, Blot S, Su JB (August 2020). "Protective effects of rimeporide on left ventricular function in golden retriever muscular dystrophy dogs" (PDF). International Journal of Cardiology. 312: 89–95. doi:10.1016/j.ijcard.2020.03.031. PMID 32199683. S2CID 214617920.
- Previtali SC, Gidaro T, Díaz-Manera J, Zambon A, Carnesecchi S, Roux-Lombard P, Spitali P, Signorelli M, Szigyarto CA, Johansson C, Gray J, Labolle D, Porte Thomé F, Pitchforth J, Domingos J, Muntoni F (September 2020). "Rimeporide as a first- in-class NHE-1 inhibitor: Results of a phase Ib trial in young patients with Duchenne Muscular Dystrophy". Pharmacological Research. 159: 104999. doi:10.1016/j.phrs.2020.104999. PMC 7482441. PMID 32535224.
- Ghaleh B, Barthélemy I, Wojcik J, Sambin L, Bizé A, Hittinger L, et al. (August 2020). "Protective effects of rimeporide on left ventricular function in golden retriever muscular dystrophy dogs" (PDF). International Journal of Cardiology. 312: 89–95. doi:10.1016/j.ijcard.2020.03.031. PMID 32199683. S2CID 214617920.