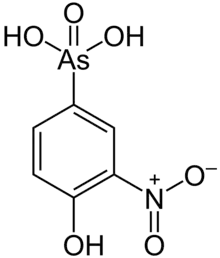
Roxarsone
Roxarsone is an organoarsenic compound that has been used in poultry production as a feed additive to increase weight gain and improve feed efficiency, and as a coccidiostat.[1] As of June 2011, it was approved for chicken feed in the United States, Canada, Australia, and 12 other countries.[2] The drug was also approved in the United States and elsewhere for use in pigs.[1][2]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(4-Hydroxy-3-nitrophenyl)arsonic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 1976533 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.049 | ||
| EC Number |
| ||
| 1221211 | |||
| KEGG | |||
| MeSH | Roxarsone | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3465 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C 6AsNH 6O 6 | |||
| Molar mass | 263.0365 g mol−1 | ||
| Melting point | > 300 °C (572 °F; 573 K) | ||
| Hazards | |||
| GHS labelling: | |||
  | |||
| Danger | |||
| H301, H331, H410 | |||
| P261, P273, P301+P310, P311, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Its use in the United States was voluntarily ended by the manufacturers in June 2011 and has been illegal since 2013.[3][4] Its use was immediately suspended in Malaysia.[5] It was banned in Canada in August 2011.[6] In Australia, its use in chicken feed was discontinued in 2012.[7] Roxarsone has been banned in the European Union since 1999.[8]
Production and applications
Roxarsone is a derivative of phenylarsonic acid (C6H5As(O)(OH)2). It was first reported in a 1923 British patent that described the nitration and diazotization of arsanilic acid.[9] When blended with calcite powder, it is used in poultry feed premixes in some parts of the world. Where available, it can be purchased in 5%, 20% and 50% concentrations.
Roxarsone was marketed as 3-Nitro by Zoetis, a former subsidiary of Pfizer now a publicly traded company. In 2006, approximately one million kilograms of roxarsone were produced in the U.S.[10]
Marketing approval in the United States
Roxarsone is one of four arsenical animal drugs that had been approved by the U.S. Food and Drug Administration (FDA) for use in poultry and swine, along with nitarsone, arsanilic acid, and carbarsone.[1] In September 2013, the FDA announced that Zoetis and Fleming Laboratories would voluntarily withdraw current roxarsone, arsanilic acid, and carbarsone approvals, leaving only nitarsone approvals in place.[3] In 2015, the FDA withdrew the approval of using nitarsone in animal feeds. The ban came into effect at the end of 2015.[11]
Controversy
Roxarsone has attracted attention as a source of arsenic contamination of poultry and other foods.[12][13] In June 2011, the manufacturers suspended sales of roxarsone in the U.S. and Canada in response to a study by the Food and Drug Administration (FDA).[14][15] The FDA found that roxarsone use was associated with elevated levels of inorganic arsenic in chicken livers.[16] An FDA press release stated that the findings raised "concerns of a very low but completely avoidable exposure to a carcinogen."[14]
A 2013 market basket study conducted in the United States linked the use of roxarsone and other arsenical feed additives to increased levels of inorganic arsenic in chicken breast meat, albeit at concentrations well below danger levels set in federal safety standards.[17][18] Breast meat from chickens exposed to arsenical feed additives contained inorganic arsenic at the level of about two parts per billion. Organic chickens not exposed to arsenical feed additives contained about half a part per billion. Federal standards permitted concentrations of 500 parts per billion of total arsenic. The study was performed on chickens raised prior to the voluntary withdrawal of Roxarsone from the market by its manufacturer in June 2011.
References
- U.S. Food and Drug Administration (June 8, 2011). "Questions and Answers Regarding 3-Nitro (Roxarsone)".
- Harris, Gardiner; Grady, Denise (9 June 2011). "Pfizer Suspends Sales of Chicken Drug". The New York Times. Retrieved 19 October 2018.
- U.S. Food and Drug Administration (September 20, 2011). "FDA Response to Citizen Petition on Arsenic-based Animal Drugs".
- "Arsenic in Chicken: Does chicken meat contain arsenic?". Chicken Check In. 21 July 2017. Retrieved 20 March 2018.
- "Malaysian Pharmaceutical Society". Malaysian Pharmaceutical Society. Retrieved 19 October 2018.
- "Sales halted after arsenic found in chicken drug". The Globe and Mail. 3 May 2018. Retrieved 19 October 2018.
- "Roxarsone not used in the Australian chicken industry" (PDF). Australian Meat Chicken Federation. 24 May 2018. Retrieved 19 October 2018.
- Philpott, Tom (11 June 2011). "Some Arsenic With That Supermarket Chicken?". Mother Jones. Retrieved 19 October 2018.
- GB 226255 19230718
- Hileman, B. (April 9, 2007). "Arsenic in Chicken Production". Chemical and Engineering News. pp. 34–35.
- U.S. Food and Drug Administration (April 1, 2015). "FDA Announces Pending Withdrawal of Approval of Nitarsone".
- Institute for Agriculture and Trade Policy (April 4, 2006). "Playing Chicken: Avoiding Arsenic in Your Meat".
{{cite news}}:|author=has generic name (help) - Consumer Reports (November 2012). "Arsenic in your food".
- U.S. Food and Drug Administration (June 8, 2011). "FDA: Pfizer will voluntarily suspend sale of animal drug 3-Nitro".
- AGCanada (7 July 2011). "Poultry antibiotic pulled in Canada". AGCanada. Retrieved 19 October 2018.
- Cevallos, M. (June 9, 2011). "Arsenic-containing drug in chicken feed to be pulled from U.S". LA Times.
- KE Nachman; PA Baron; G Raber; KA Francesconi; A Navas-Acien; DC Love (2013). "Roxarsone, Inorganic Arsenic, and Other Arsenic Species in Chicken: A U.S.-Based Market Basket Sample" (PDF). Environmental Health Perspectives. 121 (7): 818–824. doi:10.1289/ehp.1206245. PMC 3701911. PMID 23694900.
- Sabrina Tavernise (May 11, 2013). "Study Finds an Increase in Arsenic Levels in Chicken". New York Times.
Further reading
- J. R. Garbarino; A. J. Bednar; D. W. Rutherford; R. S. Beyer & R. L. Wershaw (2003). "Environmental Fate of Roxarsone in Poultry Litter. I. Degradation of Roxarsone during Composting". Environ. Sci. Technol. 37 (8): 1509–1514. Bibcode:2003EnST...37.1509G. doi:10.1021/es026219q. PMID 12731831.
- Chiou P. W.-S.; Chen K.-L.; Yu B. (1997). "Effects of roxarsone on performance, toxicity, tissue accumulation and residue of eggs and excreta in laying". Journal of the Science of Food and Agriculture. 74 (2): 229–236. doi:10.1002/(SICI)1097-0010(199706)74:2<229::AID-JSFA793>3.0.CO;2-F.
- R. L. Wershaw; J. R. Garbarino & M. R. Burkhardt (1999). Roxarsone in Natural Water Systems (PDF). Effects of Confined Animal Feeding Operations (CAFOs) on Hydrologic Resources and the Environment. Fort Collins, Colorado: U.S. Geological Survey. Retrieved 2020-06-06.
- KB Kerr; JR Narveson; FA Lux (1969). "Toxicity of an organic arsenical, 3-nitro-4-hydroxyphenylarsonic acid. Residues in chicken tissues". Journal of Agricultural and Food Chemistry. 17 (6): 1400. doi:10.1021/jf60166a021.