Rule of mixtures
In materials science, a general rule of mixtures is a weighted mean used to predict various properties of a composite material .[2][3][4] It provides a theoretical upper- and lower-bound on properties such as the elastic modulus, ultimate tensile strength, thermal conductivity, and electrical conductivity.[4] In general there are two models, one for axial loading (Voigt model),[3][5] and one for transverse loading (Reuss model).[3][6]
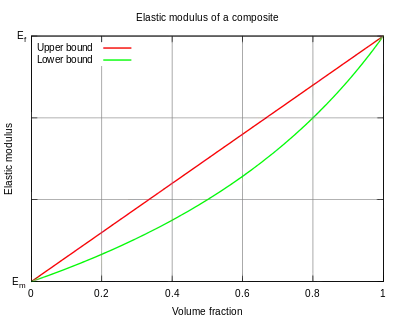
In general, for some material property (often the elastic modulus[2]), the rule of mixtures states that the overall property in the direction parallel to the fibers may be as high as
where
- is the volume fraction of the fibers
- is the material property of the fibers
- is the material property of the matrix
It is a common mistake to believe that this is the upper-bound modulus for Young's modulus. The real upper-bound Young's modulus is larger than given by this formula. Even if both constituents are isotropic, the real upper bound is plus a term in the order of square of the difference of the Poisson's ratios of the two constituents.[1]
The inverse rule of mixtures states that in the direction perpendicular to the fibers, the elastic modulus of a composite can be as low as
If the property under study is the elastic modulus, this quantity is called the lower-bound modulus, and corresponds to a transverse loading.[3]
Derivation for elastic modulus
Voigt Modulus
Consider a composite material under uniaxial tension . If the material is to stay intact, the strain of the fibers, must equal the strain of the matrix, . Hooke's law for uniaxial tension hence gives
-
(1)
where , , , are the stress and elastic modulus of the fibers and the matrix, respectively. Noting stress to be a force per unit area, a force balance gives that
-
(2)
where is the volume fraction of the fibers in the composite (and is the volume fraction of the matrix).
If it is assumed that the composite material behaves as a linear-elastic material, i.e., abiding Hooke's law for some elastic modulus of the composite and some strain of the composite , then equations 1 and 2 can be combined to give
Finally, since , the overall elastic modulus of the composite can be expressed as[7]
Reuss modulus
Now let the composite material be loaded perpendicular to the fibers, assuming that . The overall strain in the composite is distributed between the materials such that
The overall modulus in the material is then given by
since , .[7]
Other properties
Similar derivations give the rules of mixtures for
- mass density:where f is the atomic percent of fiber in the mixture.
- ultimate tensile strength:
- thermal conductivity:
- electrical conductivity:
See also
When considering the empirical correlation of some physical properties and the chemical composition of compounds, other relationships, rules, or laws, also closely resembles the rule of mixtures:
- Amagat's law – Law of partial volumes of gases
- Gladstone–Dale equation – Optical analysis of liquids, glasses and crystals
- Kopp's law – Uses mass fraction
- Kopp–Neumann law – Specific heat for alloys
- Vegard's law – Crystal lattice parameters
References
- Yu, Wenbin (2016). An Introduction to Micromechanics. Switzerland: Trans Tech Publications. pp. 3–24. ISBN 9783038357469.
- Alger, Mark. S. M. (1997). Polymer Science Dictionary (2nd ed.). Springer Publishing. ISBN 0412608707.
- "Stiffness of long fibre composites". University of Cambridge. Retrieved 1 January 2013.
- Askeland, Donald R.; Fulay, Pradeep P.; Wright, Wendelin J. (2010-06-21). The Science and Engineering of Materials (6th ed.). Cengage Learning. ISBN 9780495296027.
- Voigt, W. (1889). "Ueber die Beziehung zwischen den beiden Elasticitätsconstanten isotroper Körper". Annalen der Physik. 274 (12): 573–587. Bibcode:1889AnP...274..573V. doi:10.1002/andp.18892741206.
- Reuss, A. (1929). "Berechnung der Fließgrenze von Mischkristallen auf Grund der Plastizitätsbedingung für Einkristalle". Zeitschrift für Angewandte Mathematik und Mechanik. 9 (1): 49–58. Bibcode:1929ZaMM....9...49R. doi:10.1002/zamm.19290090104.
- "Derivation of the rule of mixtures and inverse rule of mixtures". University of Cambridge. Retrieved 1 January 2013.