Salvinorin
Salvinorins are a group of natural chemical compounds and their structural analogs. Several salvinorins have been isolated from Salvia divinorum. They are classified as diterpenoid furanolactones. Salvinorin A is a hallucinogen with dissociative effects.
Several salvinorins have been isolated and characterized.
| Name | Structure | R1 | R2 | Chemical formula | Molar mass | CAS number | PubChem |
|---|---|---|---|---|---|---|---|
| Salvinorin A |  | –OCOCH3 | − | C23H28O8 | 432.46 g·mol−1 | 83729-01-5 | CID 128563 from PubChem |
| Salvinorin B | –OH | − | C21H26O7 | 390.43 g·mol−1 | 92545-30-7 | CID 11440685 from PubChem | |
| Salvinorin C |  | –OCOCH3 | –OCOCH3 | C25H30O9 | 475.29 g·mol−1 | 385785-99-9 | – |
| Salvinorin D[1] | –OH | –OCOCH3 | C23H28O8 | 432.47 g·mol−1 | 540770-13-6 | – | |
| Salvinorin E[1] | –OCOCH3 | –OH | C23H28O8 | 432.47 g·mol−1 | 540770-14-7 | – | |
| Salvinorin F[1] | –H | –OH | C21H26O6 | 374.43 g·mol−1 | 540770-15-8 | – | |
| Salvinorin G | =O | –OCOCH3 | C23H26O8 | 430.45 g·mol−1 | 866622-54-0 | – | |
| Salvinorin H | –OH | –OH | C21H26O7 | 390.43 g·mol−1 | 872004-62-1 | – | |
| Salvinorin I |  | – | – | C21H28O7 | 392.45 g·mol−1 | 917951-71-4 | – |
| 17α-Salvinorin J |  | – | – | C23H30O8 | 434.49 g·mol−1 | 1157894-83-1 | – |
| 17β-Salvinorin J | 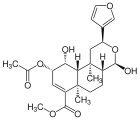 | – | – | C23H30O8 | 434.49 g·mol−1 | 1157894-85-3 | – |
Occurrence
Originally isolated from S. divinorum, salvinorins are also detected in smaller amounts in:
- Salvia recognita (salvinorin A, 212.9 μg/g)[2]
- Salvia absconditiflora (salvinorin A at 51.5 μg/g, and salvinorin B at 402.2 μg/g)[2]
- Salvia glutinosa (salvinorin A, 38.9 μg/g)[2]
- Salvia potentillifolia (salvinorin B, 2352.0 μg/g)[2]
- Salvia adenocaulon (salvinorin B, 768.8 μg/g)[2]
For comparison, the amount of salvinorin A in S. divinorum ranges from 0.89 to 3.70 mg/g. All fractions reported are based on dry mass.[2]
Interestingly, the above reported species are not very closely related to S. divinorum.[2]
Associated compounds
In search for useful biological activity, several synthetic and semi-synthetic analogs have been prepared for study. Semi-synthetic analogs include salvinorin B ethoxymethyl ether and salvinorin B methoxymethyl ether. Fully synthetic analogs include herkinorin.
Several derivates can be conveniently made from salvinorin B. Most derivatives are selective kappa opioid agonists as with salvinorin A, although some are even more potent, with the most potent compound 2-ethoxymethyl salvinorin B being ten times stronger than salvinorin A. Some derivatives, such as herkinorin, reduce kappa opioid action and instead act as mu opioid agonists.[3][4][5][6]
22-Thiocyanato-salvinorin A is notable because of its functional selectivity.[7] 2-Methoxymethyl Salvinorin B is seven times more potent than Salvinorin A at KOPr in GTP-γS assays.[8]
Many other terpenoids have been isolated from Salvia divinorum, including classes named divinatorins and salvinicins. None of these compounds have shown significant (sub-micromolar) affinity at the kappa-opioid receptor, and there is no evidence that they contribute to the plant's psychoactivity.[9][1]
References
- Munro TA; Rizzacasa MA (2003). "Salvinorins D-F, new neoclerodane diterpenoids from Salvia divinorum, and an improved method for the isolation of salvinorin A". Journal of Natural Products. 66 (5): 703–5. doi:10.1021/np0205699. PMID 12762813.
- Hatipoglu, SD; Yalcinkaya, B; Akgoz, M; Ozturk, T; Goren, AC; Topcu, G (November 2017). "Screening of Hallucinogenic Compounds and Genomic Characterisation of 40 Anatolian Salvia Species". Phytochemical Analysis. 28 (6): 541–549. doi:10.1002/pca.2703. PMID 28722248.
- Munro TA; Duncan KK; Xu W; Wang Y; Liu-Chen LY; Carlezon WA; Cohen BM; Béguin C (2008). "Standard protecting groups create potent and selective κ opioids: salvinorin B alkoxymethyl ethers". Bioorganic & Medicinal Chemistry. 16 (3): 1279–86. doi:10.1016/j.bmc.2007.10.067. PMC 2568987. PMID 17981041.
- Holden KG; Tidgewell K; Marquam A; Rothman RB; Navarro H; Prisinzano TE (2007). "Synthetic studies of neoclerodane diterpenes from Salvia divinorum: exploration of the 1-position". Bioorganic & Medicinal Chemistry Letters. 17 (22): 6111–5. doi:10.1016/j.bmcl.2007.09.050. PMC 2111044. PMID 17904842.
- Lee DY; He M; Liu-Chen LY; Wang Y; Li JG; Xu W; Ma Z; Carlezon WA; Cohen B (2006). "Synthesis and in vitro pharmacological studies of new C(4)-modified salvinorin A analogues". Bioorganic & Medicinal Chemistry Letters. 16 (21): 5498–502. doi:10.1016/j.bmcl.2006.08.051. PMID 16945525.
- Béguin C; Richards MR; Li JG; Wang Y; Xu W; Liu-Chen LY; Carlezon WA; Cohen BM (2006). "Synthesis and in vitro evaluation of salvinorin A analogues: effect of configuration at C(2) and substitution at C(18)". Bioorganic & Medicinal Chemistry Letters. 16 (17): 4679–85. doi:10.1016/j.bmcl.2006.05.093. PMID 16777411.
- White K, Robinson JE, Zhu H, et al. (2014). "The G-protein biased k-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo". J. Pharmacol. Exp. Ther. 352 (1): 98–109. doi:10.1124/jpet.114.216820. PMC 4279099. PMID 25320048.
- Wang, Y.; Chen, Y.; Xu, W.; Lee, D.; Ma, Z; Rawls, S.; Cowan, A.; Liu-Chen, L. (2008). "2-methoxymethyl-salvinorin B is a potent kappa opioid receptor agonist with longer lasting action in vivo than salvinorin A." Journal of Pharmacology and Experimental Therapeutics. 324 (3): 1073–1083. doi:10.1124/jpet.107.132142. PMC 2519046. PMID 18089845.
- Bigham AK; Munro TA; Rizzacasa MA; Robins-Browne RM (2003). "Divinatorins A-C, new neoclerodane diterpenoids from the controlled sage Salvia divinorum". Journal of Natural Products. 66 (9): 1242–4. CiteSeerX 10.1.1.693.6690. doi:10.1021/np030313i. PMID 14510607.