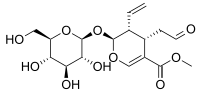
Secologanin
Secologanin is a secoiridoid monoterpene synthesized from geranyl pyrophosphate in the mevalonate pathway. Secologanin then proceeds with dopamine or tryptamine to form ipecac and terpene indole alkaloids, respectively.
 | |
| Names | |
|---|---|
| IUPAC name
Methyl (2S,3R,4S)-3-ethenyl-2-(β-D-glucopyranosyloxy)-4-(2-oxoethyl)-3,4-dihydro-2H-pyran-5-carboxylate | |
| Systematic IUPAC name
Methyl (4S,5R,6S)-3-ethenyl-4-(2-oxoethyl)-2-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,4-dihydro-2H-pyran-5-carboxylate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H24O10 | |
| Molar mass | 388.369 g·mol−1 |
| Density | 1.42 g/mL |
| Boiling point | 595.5 °C (1,103.9 °F; 868.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Biosynthesis
Secologanin biosynthesis begins from geranyl pyrophosphate (GPP) taken from the mevalonate pathway used to make terpenoids. Recent efforts have characterized the entire secologanin biosynthetic pathway.[1] Secologanin is formed from loganin through the action of the enzyme secologanin synthase. Secologanin is then able to proceed onto produce ipecac and terpene indole alkaloids.[2]
References
- Miettinen, Karel; Dong, Lemeng; Navrot, Nicolas; Schneider, Thomas; Burlat, Vincent; Pollier, Jacob; Woittiez, Lotte; van der Krol, Sander; Lugan, Raphaël; Ilc, Tina; Verpoorte, Robert; Oksman-Caldentey, Kirsi-Marja; Martinoia, Enrico; Bouwmeester, Harro; Goossens, Alain; Memelink, Johan; Werck-Reichhart, Danièle (7 April 2014). "The seco-iridoid pathway from Catharanthus roseus". Nature Communications. 5 (1): 3606. doi:10.1038/ncomms4606. PMC 3992524. PMID 24710322.
- "Secologanin Biosynthesis". Retrieved 31 May 2011.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.