Semi-solid flow battery
A semi-solid flow battery is a type of flow battery using solid battery active materials or involving solid species in the energy carrying fluid. A research team in MIT proposed this concept using lithium-ion battery materials.[2] In such a system, both positive (cathode) and negative electrode (anode) consist of active material particles with carbon black suspended in liquid electrolyte. Active material suspensions are stored in two energy storage tanks. The suspensions are pumped into the electrochemical reaction cell when charging and discharging. This design takes advantage of both the designing flexibility of flow batteries and the high energy density active materials of lithium-ion batteries.
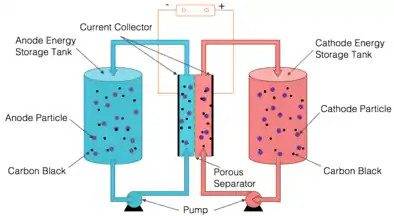
Flow Mode and Chemistries
Two different flow modes were explored, intermittent flow mode and continuous flow mode. In an intermittent flow mode, suspensions are pumped into the electrochemical reaction cell in a batch basis and a new batch is pumped in only after the previous batch has been fully charged/discharged. In a continuous flow mode, however, the suspensions are continuously pumped through the electrochemical reaction cell during the charge/discharge process. By using lithium-ion battery active materials, the energy density of the flow battery system can be significantly improved. An aqueous system was also demonstrated besides the organic one.[3] Other chemistries have also been explored for this system, such as sodium-ion battery, lithium-sulfur battery, and others.
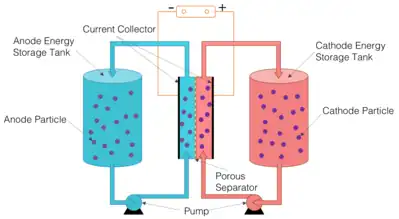
System Developments
Solid Dispersion Flow Battery
Despite the significant advantage of such a system, one key limitation was the high viscosity, which makes the power consumption for pumping very high, hence decreasing the energy efficiency. Another research team in University of Virginia reported a carbon-free flow battery system.[5] In this new system, also called Solid Dispersion Flow Battery, a new reaction mechanism was discovered with electrochemical reactions occurring based on particle collisions.[6]
Redox Targeting Flow Battery
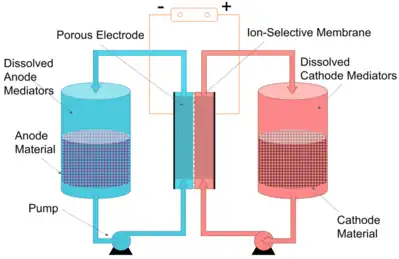
Another approach is to pump the liquid phase only, leaving the solid active materials in the energy storage tanks. A research group reported redox targeting flow battery.[8] There are redox targeting materials dissolved in the electrolyte and electrochemical reactions occur between the dissolved species. The solid materials are then been chemically oxidized or reduced. By keeping solid materials in the tanks, only the liquid electrolyte is being pumped. This saves the pumping energy although the voltage efficiency is sacrificed.[9]
References
- Qi, Zhaoxiang; Koenig, Gary M. (July 2017). "Review Article: Flow battery systems with solid electroactive materials". Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena. 35 (4): 040801. Bibcode:2017JVSTB..35d0801Q. doi:10.1116/1.4983210. ISSN 2166-2746.
- Duduta, Mihai; Ho, Bryan; Wood, Vanessa C.; Limthongkul, Pimpa; Brunini, Victor E.; Carter, W. Craig; Chiang, Yet-Ming (2011-05-20). "Semi-Solid Lithium Rechargeable Flow Battery". Advanced Energy Materials. 1 (4): 511–516. doi:10.1002/aenm.201100152. ISSN 1614-6832. S2CID 97634258.
- Li, Zheng; Smith, Kyle C.; Dong, Yajie; Baram, Nir; Fan, Frank Y.; Xie, Jing; Limthongkul, Pimpa; Carter, W. Craig; Chiang, Yet-Ming (2013). "Aqueous semi-solid flow cell: demonstration and analysis". Physical Chemistry Chemical Physics. 15 (38): 15833–9. Bibcode:2013PCCP...1515833L. doi:10.1039/C3CP53428F. ISSN 1463-9076. PMID 23995625.
- Qi, Zhaoxiang; Koenig, Gary M. (July 2017). "Review Article: Flow battery systems with solid electroactive materials". Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena. 35 (4): 040801. Bibcode:2017JVSTB..35d0801Q. doi:10.1116/1.4983210. ISSN 2166-2746.
- Qi, Zhaoxiang; Koenig, Gary M. (2016-08-15). "A carbon-free lithium-ion solid dispersion redox couple with low viscosity for redox flow batteries". Journal of Power Sources. 323: 97–106. Bibcode:2016JPS...323...97Q. doi:10.1016/j.jpowsour.2016.05.033. ISSN 0378-7753.
- Qi, Zhaoxiang; Dong, Hongxu; Koenig, Gary M. (2017-11-01). "Electrochemical Characterization of Lithium-Ion Battery Cathode Materials with Aqueous Flowing Dispersions". Electrochimica Acta. 253: 163–170. doi:10.1016/j.electacta.2017.09.031. ISSN 0013-4686.
- Qi, Zhaoxiang; Koenig, Gary M. (July 2017). "Review Article: Flow battery systems with solid electroactive materials". Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena. 35 (4): 040801. Bibcode:2017JVSTB..35d0801Q. doi:10.1116/1.4983210. ISSN 2166-2746.
- Huang, Qizhao; Yang, Jing; Ng, Chee Boon; Jia, Chuankun; Wang, Qing (2016). "A redox flow lithium battery based on the redox targeting reactions between LiFePO4 and iodide". Energy & Environmental Science. 9 (3): 917–921. doi:10.1039/C5EE03764F. ISSN 1754-5692.
- Qi, Zhaoxiang; Koenig, Gary M. (July 2017). "Review Article: Flow battery systems with solid electroactive materials". Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena. 35 (4): 040801. Bibcode:2017JVSTB..35d0801Q. doi:10.1116/1.4983210. ISSN 2166-2746.