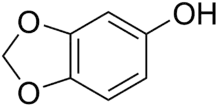
Sesamol
Sesamol is a natural organic compound which is a component of sesame seeds and sesame oil, with anti-inflammatory, antioxidant, antidepressant and neuroprotective properties. It is a white crystalline solid that is a derivative of phenol. It is sparingly soluble in water, but miscible with most oils. It can be produced by organic synthesis from heliotropine.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2H-1,3-Benzodioxol-5-ol | |
| Other names
1,3-Benzodioxol-5-ol Benzo[d][1,3]dioxol-5-ol Sesamol 3,4-Methylenedioxyphenol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.784 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H6O3 | |
| Molar mass | 138.12 g/mol |
| Melting point | 62 to 65 °C (144 to 149 °F; 335 to 338 K) |
| Boiling point | 121 to 127 °C (250 to 261 °F; 394 to 400 K) at 5 mmHg |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Sesamol has been found to be an antioxidant that may prevent the spoilage of oils.[2][3] It also may prevent the spoilage of oils by acting as an antifungal.[4] It can be used in the synthesis of paroxetine.[5]: 138–141
Sesamol's molecular targets and mechanism of action, at least for its antidepressant-like effects, is found to be through the brain nerve growth factor (NGF) and endocannabinoid signalling under the regulatory drive of the CB1 receptors.[6]
Alexander Shulgin used sesamol in his book PiHKAL to make MMDA-2.
References
- Sesamol Archived 2010-01-14 at the Wayback Machine at Chemicalland21.com
- Kim JY, Choi DS, Jung MY (May 2003). "Antiphoto-oxidative activity of sesamol in methylene blue- and chlorophyll-sensitized photo-oxidation of oil". Journal of Agricultural and Food Chemistry. 51 (11): 3460–5. doi:10.1021/jf026056p. PMID 12744684.
- Ohsawa T (1991). "Sesamol and sesaminol as antioxidants". New Food Industry. 33 (6): 1–5.
- Wynn JP, Kendrick A, Ratledge C (June 1997). "Sesamol as an inhibitor of growth and lipid metabolism in Mucor circinelloides via its action on malic enzyme". Lipids. 32 (6): 605–10. doi:10.1007/s11745-997-0077-1. PMID 9208389. S2CID 4015004.
- Li JJ (2004). Contemporary drug synthesis. Hoboken, N.J.: Wiley. ISBN 978-0-471-21480-9.
- Hassanzadeh P, Hassanzadeh A (October 2013). "Implication of NGF and endocannabinoid signaling in the mechanism of action of sesamol: a multi-target natural compound with therapeutic potential". Psychopharmacology. 229 (4): 571–578. doi:10.1007/s00213-013-3111-z. PMID 23624775. S2CID 253748878.
