Zoster vaccine
A zoster vaccine is a vaccine that reduces the incidence of herpes zoster (shingles), a disease caused by reactivation of the varicella zoster virus, which is also responsible for chickenpox.[7] Shingles provokes a painful rash with blisters, and can be followed by chronic pain (postherpetic neuralgia), as well as other complications. Older people are more often affected, as are people with weakened immune systems (immunosuppression). Both shingles and postherpetic neuralgia can be prevented by vaccination.[8]
 | |
| Vaccine description | |
|---|---|
| Target | Herpes zoster, postherpetic neuralgia, Ramsay Hunt syndrome type II |
| Vaccine type |
|
| Clinical data | |
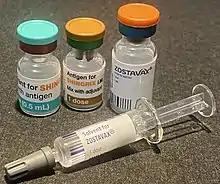
| Trade names | Zostavax, Shingrix |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
|
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| (verify) | |
Two zoster vaccines have been approved for use in people over 50 years old.[8] Shingrix (GSK) is a recombinant subunit vaccine which has been used in many countries since 2017.[9] Zostavax (Merck), in use since 2006,[10] is an attenuated vaccine which consists of a larger-than-normal dose of chickenpox vaccine.[7] Unlike Shingrix, Zostavax is not suitable for people with immunosuppression or diseases that affect the immune system.[8] Zostavax was discontinued in the United States in November 2020.[11]
Shingrix appears to prevent more cases of shingles than Zostavax, although side effects seem to be more frequent.[9][12]
Another vaccine, known as varicella vaccine, is used to prevent diseases caused by the same virus.[13]
Medical uses
Zoster vaccination is used to prevent shingles and its complications, including postherpetic neuralgia.[7][8] It can be considered a therapeutic vaccine, given that it is used to treat a latent virus that has remained dormant in cells since chicken pox infection earlier in life.[7] The two available zoster vaccines are intended for use in people over the age of 50.[8] As of 2021 it remained to be confirmed whether a booster dose was required,[14][9] but the Advisory Committee on Immunization Practices (ACIP) in the United States recommends Shingrix for adults over the age of 50, including those who have already received Zostavax.[15]
Shingrix

The ACIP voted that Shingrix is preferred over Zostavax for the prevention of zoster and related complications because data showed vaccine efficacy of more than 90% against shingles across all age groups. Unlike Zostavax, which is given as a single shot, Shingrix is given as two intramuscular doses, two to six months apart.[15][10] Shingrix provides high levels of immunity for at least 7 years after vaccination, but it is possible the vaccine may provide protection for much longer.[16][17]
A large randomized clinical trial showed Shingrix reduced the incidence of shingles 96.6% in the 50–59 age group, and 91.3% in those over age 70.[10] The absolute decrease in risk of herpes zoster following immunization over three and a half years is 3.3% (3.54% down to 0.28%) while the decrease in the risk of postherpetic neuralgia is 0.3% (0.34% down to 0.06%).[18]
Zostavax
_vaccine_(live))_(United_Kingdom).jpg.webp)
The Zostavax vaccine (both single dose and two-dose regime) is likely effective at protecting people from herpes zoster disease for a duration of up to three years.[19] The degree of longer term protection (beyond 4 years from the initial vaccination) is not clear. The need for re-vaccination after the first full vaccine schedule is complete remains to be confirmed.[19]
Zostavax was shown to reduce the incidence of shingles by 51% in a study of 38,000 adults aged 60 and older who received the vaccine. The vaccine also reduced by 67% the number of cases of postherpetic neuralgia (PHN) and reduced the severity and duration of pain and discomfort associated with shingles, by 61%.[20][21][3] The FDA originally recommended it for individuals 60 years of age or older who are not severely allergic to any of its components and who meet the following requirements:[22][23]
- do not have a weakened immune system due to HIV/AIDS or another disease or medications (such as steroids, radiation and chemotherapy) that affect the immune system;
- do not have a history of cancer affecting the bone marrow or lymphatic system, such as leukemia or lymphoma; and
- do not have active, untreated tuberculosis.
In 2006, the US Advisory Committee on Immunization Practices (ACIP) recommended that the live vaccine be given to all adults age 60 and over, including those who have had a previous episode of shingles,[24] and those who do not recall having had chickenpox, since more than 99% of Americans ages 40 and older have had chickenpox.[16]
Side effects
Shingrix
Temporary side effects from the Shingrix shots are likely and can be severe enough in one out of six people to affect normal daily activities for up to three days.[16] Mild to moderate pain at the injection site is common, and some may have redness or swelling.[16] Side effects include fatigue, muscle pain, headache, shivering, fever, and nausea.[16] Symptoms usually resolve in two to three days.[16] Side effects with Shingrix are greater than those with Zostavax and occur more frequently in individuals aged 50 to 69 years compared with those 70 years and older.[9][4]
Zostavax
The live vaccine (Zostavax) is very safe; one to a few percent of people develop a mild form of chickenpox, often with about five or six blisters around the injection site, and without fever. The blisters are harmless and temporary.[25][26] In one study 64% of the Zostavax group and 14% of the controls had some adverse reaction. However, the rates of serious adverse events were comparable between the Zostavax group (0.6%) and those receiving the placebo (0.5%).[27] A study including children with leukaemia found that the risk of getting shingles after vaccination is much lower than the risk of getting shingles for children with natural chicken pox in their history. Data from healthy children and adults point in the same direction.[25]
Zostavax is not used in people with compromised immune function.[28][29]
Composition
Shingrix
Shingrix is a suspension for intramuscular injection consisting of a lyophilized recombinant varicella zoster virus glycoprotein E antigen that is reconstituted at the time of use with AS01B suspension as an immunological adjuvant. The antigen is a purified truncated form of the glycoprotein, expressed in Chinese hamster ovary cells. The AS01B adjuvant suspension is composed of 3-O-desacyl-4'-monophosphoryl lipid A (MPL) from Salmonella (Minnesota strain) and a saponin molecule (QS-21) purified from Quillaja saponaria (soap bark tree) extract, combined in a liposomal formulation consisting of dioleoyl phosphatidylcholine (DOPC) and cholesterol in phosphate-buffered saline solution.[30]
Zostavax
Zostavax contains live attenuated varicella-zoster virus.[25][11] It is injected subcutaneously (under the skin) in the upper arm.[31] The live vaccine is produced using the MRC-5 line of fetal cells.[3] This has raised religious and ethical concerns for some potential users, since that cell line was derived from an aborted fetus.[32]
Cost effectiveness
A 2007 study found that the live vaccine is likely to be cost-effective in the US, projecting an annual savings of US$82 to US$103 million in healthcare costs with cost-effectiveness ratios ranging from US$16,229 to US$27,609 per quality-adjusted life year gained.[33] In 2007, the live vaccine was officially recommended in the US for healthy adults aged 60 and over, but is no longer given out in the United States as of 2020, given the superiority of Shingrix.[34][35][36]
In Canada the cost of Shingrix is about CA$300 for the two doses.[18] This likely represents a more cost effective intervention than the live vaccine given its lower cost and increased effectiveness.[37]
History
European Union
In 2006, the European Medicines Agency (EMA) issued a marketing authorization for the zoster vaccine to Sanofi Pasteur for routine vaccination in individuals aged 60 and over.[38][5] In 2007, the EMA updated the marketing authorization for routine vaccination in individuals aged 50 and over.[39][5]
Shingrix was approved for medical use in the European Union in March 2018, with an indication for the prevention of herpes zoster (HZ) and post-herpetic neuralgia (PHN) in adults 50 years of age or older.[6]
United Kingdom
From 2013, the UK National Health Service (NHS) started offering shingles vaccination to elderly people. People aged either 70 or 79 on 1 September 2013, were offered the vaccine. People aged 71 to 78 on that date would only have an opportunity to have the shingles vaccine after reaching the age of 79.[40] The original intention was for people aged between 70 and 79 to be vaccinated, but the NHS later said that the vaccination program was being staggered as it would be impractical to vaccinate everyone in their 70s in a single year.[41]
In 2021, vaccination against shingles is available on the NHS to people aged 70 to 79.[42] Vaccination is with single-dose Zostavax, except for people for whom Zostavax is deemed unsuitable, for example, with a condition that affects the immune system, for whom two-dose Shingrix vaccine is recommended.[42] The NHS stated "The shingles vaccine is not available on the NHS to anyone aged 80 or over because it seems to be less effective in this age group".[42]
United States
Zostavax was developed by Merck & Co. and approved and licensed by the US Food and Drug Administration (FDA) in May 2006,[20] In 2011, the FDA approved the live vaccine for use in individuals 50 to 59 years of age.[3][43] Shingrix is a zoster vaccine developed by GlaxoSmithKline that was approved in the United States in October 2017.[44] Shingrix, which provides strong protection against shingles and PHN, was preferred over Zostavax before Zostavax was discontinued.[45]
In June 2020, Merck discontinued the sale of Zostavax in the US. Vaccine doses already held by practitioners could still be administered up to the expiration date (none expired later than November 2020).[46][11]
The US Centers for Disease Control and Prevention (CDC) recommends that healthy adults 50 years and older get two doses of Shingrix, at least two months apart. Initial clinical trials only tested a gap of less than six months between doses, but unexpected popularity and resulting shortages caused further testing to validate wider spacing of the two doses.[47][48] Adults 19 years and older who are immunocompromised because of disease or therapy are also recommended to receive two doses of Shingrix.[16]
The zoster vaccine is covered by Medicare Part D. In 2019, more than 90% of Medicare Part D vaccine spending was for the zoster vaccine. 5.8 million vaccine doses were administered to Part D beneficiaries that year at a cost of $857 million.[49]
References
- "Zoster vaccine live (Zostavax) Use During Pregnancy". Drugs.com. 3 January 2020. Retrieved 23 January 2020.
- "Zostavax vaccine – Summary of Product Characteristics (SmPC)". (emc). 28 January 2019. Retrieved 6 September 2020.
- "Zostavax – zoster vaccine live injection, powder, lyophilized, for suspension Sterile Diluent – sterile water injection". DailyMed. 26 September 2019. Retrieved 6 September 2020.
- "Shingrix- zoster vaccine recombinant, adjuvanted kit". DailyMed. 4 October 2019. Retrieved 6 September 2020.
- "Zostavax EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 31 July 2020.
- "Shingrix EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 31 July 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, et al. (July 2015). "Varicella zoster virus infection". Nature Reviews. Disease Primers. 1: 15016. doi:10.1038/nrdp.2015.16. PMC 5381807. PMID 27188665.
- Saguil A, Kane S, Mercado M, Lauters R (November 2017). "Herpes zoster and postherpetic neuralgia: prevention and management". American Family Physician. 96 (10): 656–663. PMID 29431387.
- Tricco AC, Zarin W, Cardoso R, Veroniki AA, Khan PA, Nincic V, et al. (October 2018). "Efficacy, effectiveness, and safety of herpes zoster vaccines in adults aged 50 and older: systematic review and network meta-analysis". BMJ. 363: k4029. doi:10.1136/bmj.k4029. PMC 6201212. PMID 30361202.
- Pan CX, Lee MS, Nambudiri VE (2022). "Global herpes zoster incidence, burden of disease, and vaccine availability: a narrative review". Therapeutic Advances in Vaccines and Immunotherapy. 10. doi:10.1177/25151355221084535. PMC 8941701. PMID 35340552.
- "What everyone should know about Zostavax". U.S. Centers for Disease Control and Prevention (CDC). 18 June 2018. Retrieved 16 November 2018.
- Gagliardi AM, Andriolo BN, Torloni MR, et al. (November 2019). "Vaccines for preventing herpes zoster in older adults". The Cochrane Database of Systematic Reviews. 2019 (11). doi:10.1002/14651858.CD008858.pub4. PMC 6836378. PMID 31696946.
- "Herpes Zoster Vaccination". U.S. Centers for Disease Control and Prevention (CDC). 31 July 2015. Retrieved 26 October 2021.
- "Chapter 28a: Shingles (herpes zoster)". Green Book of immunisation (PDF). 23 August 2021. p. 8.
The need for booster doses of either Shingrix and Zostavax has not yet been determined.
- Han DH (25 October 2017). "ACIP: New Vaccine Recommendations for Shingles Prevention". MPR. Retrieved 30 October 2017.
- "Shingles Vaccination: What Everyone Should Know". U.S. Centers for Disease Control and Prevention (CDC). 24 May 2022. Retrieved 1 January 2023.
- Shah RA, Limmer AL, Nwannunu CE, Patel RR, Mui UN, Tyring SK (July 2019). "Shingrix for Herpes Zoster: A Review". Skin Therapy Letter. 24 (4): 5–7. PMID 31339679.
- "[114] Shingrix: A New Vaccine for Shingles". Therapeutics Initiative. 11 October 2018. Retrieved 14 October 2018.
- de Oliveira Gomes J, Gagliardi AM, Andriolo BN, Torloni MR, Andriolo RB, Puga ME, Canteiro Cruz E, et al. (Cochrane Acute Respiratory Infections Group) (October 2023). "Vaccines for preventing herpes zoster in older adults". The Cochrane Database of Systematic Reviews. 2023 (10): CD008858. doi:10.1002/14651858.CD008858.pub5. PMC 10542961. PMID 37781954.
- Mitka M (July 2006). "FDA approves shingles vaccine: herpes zoster vaccine targets older adults". JAMA. 296 (2): 157–158. doi:10.1001/jama.296.2.157. PMID 16835412.
- "FDA Licenses New Vaccine to Reduce Older Americans' Risk of Shingles" (Press release). U.S. Food and Drug Administration (FDA). 26 May 2006. Retrieved 31 October 2009.
- "Patient Information about Zostavax". U.S. Food and Drug Administration (FDA). May 2006. Archived from the original on 19 June 2009. Retrieved 31 October 2009.
- "Patient Information about Zostavax (Text) 12/2008". U.S. Food and Drug Administration (FDA). December 2008. Archived from the original on 27 August 2009. Retrieved 31 October 2009.
- "CDC's Advisory Committee Recommends "Shingles" Vaccination" (Press release). U.S. Centers for Disease Control and Prevention (CDC). 26 October 2006. Retrieved 31 October 2009.
- "About Zostavax". Merck Sharp & Dohme Corp. May 2018. Archived from the original on 17 November 2018. Retrieved 16 November 2018.
- Harris S (18 January 2011). "Shingles Vaccine: Expert Q&A". WebMD. Retrieved 4 January 2014.
- Cunha JP. "Zostavax Side Effects Center". RxList. Retrieved 4 January 2014.
- "Zostavax vaccine". Therapeutic Goods Administration (TGA) (Press release). 7 March 2017. Retrieved 11 July 2020.
- "Zostavax vaccine". Therapeutic Goods Administration (TGA) (Press release). 6 July 2020. Retrieved 11 July 2020.
- "FDA Shingrix Briefing Document" (PDF). U.S. Food and Drug Administration (FDA). Retrieved 28 October 2017.
- "Shingles Vaccine (Zoster Shingles Vaccine Live, Zostavax)". Medicinenet.com. Retrieved 29 April 2018.
- Davidson MW (13 November 2015). "Human Fetal Lung Fibroblast Cells (MRC-5 Line)". Florida State University.
- Pellissier JM, Brisson M, Levin MJ (November 2007). "Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults". Vaccine. 25 (49): 8326–8337. doi:10.1016/j.vaccine.2007.09.066. PMID 17980938.
- Harpaz R, Ortega-Sanchez IR, Seward JF (June 2008). "Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP)" (PDF). MMWR. Recommendations and Reports. 57 (RR-5): 1–30, quiz CE2–4. PMID 18528318.
- Advisory Committee on Immunization Practices (November 2007). "Recommended adult immunization schedule: United States, October 2007-September 2008". Annals of Internal Medicine. 147 (10): 725–729. doi:10.7326/0003-4819-147-10-200711200-00187. PMID 17947396. S2CID 31630647.
- Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R (January 2018). "Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines" (PDF). MMWR. Morbidity and Mortality Weekly Report. 67 (3): 103–108. doi:10.15585/mmwr.mm6703a5. PMC 5812314. PMID 29370152.
- Le P, Rothberg MB (February 2018). "Cost-effectiveness of the Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults". JAMA Internal Medicine. 178 (2): 248–258. doi:10.1001/jamainternmed.2017.7431. PMC 5838796. PMID 29297049.
- "Zostavax EPAR" (PDF). European Medicines Agency (EMA). July 2006. Retrieved 27 March 2011.
- "Zostavax-H-C-674-II-03 Scientific Discussion" (PDF). 21 June 2007. Retrieved 27 March 2011.
- "Shingles Vaccination (16 July 2013, archived)". NHS UK. Archived from the original on 2 June 2014.
- "Who can have the shingles vaccine? (16 July 2013, archived)". NHS UK. Archived from the original on 10 April 2014.
- "Shingles vaccine overview". National Health Service (NHS). 31 August 2021. Retrieved 26 November 2021.
- "FDA approves Zostavax vaccine to prevent shingles in individuals 50 to 59 years of age" (Press release). U.S. Food and Drug Administration (FDA). 24 March 2011. Archived from the original on 27 March 2011. Retrieved 27 March 2011.
- "BBL approval" (PDF). U.S. Food and Drug Administration (FDA). 20 October 2017. Retrieved 29 April 2018.
- "Shingles (Herpes Zoster) Vaccination (as of 25 October 2018)". U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on 7 December 2018.
- "Product Discontinuation Notice: Zostavax (Zoster Vaccine Live)" (PDF). Archived from the original (PDF) on 18 August 2020.
- Cimons M (29 July 2018). "Don't panic if you're put on a waiting list for the new shingles vaccine". The Washington Post. Retrieved 24 January 2020.
The Centers for Disease Control and Prevention, which issues vaccine recommendations, says patients who wait longer than six months needn't worry, but they should get that second dose as soon as possible. Be sure not to skip it, because two doses convey the maximum immunity, more than 90%.
- "Frequently Asked Questions About Shingrix". U.S. Centers for Disease Control and Prevention (CDC). 26 March 2018. Retrieved 23 January 2020.
You and patients should make every effort to ensure that two doses are administered within the recommended 2–6 month interval. If more than 6 months have elapsed since the first dose, administer the second dose as soon as possible. Do not restart the vaccine series...
- Report to the Congress: Medicare and the Health Care Delivery System. Medicare Payment Advisory Commission. June 2021. p. 254.
Further reading
- World Health Organization (2014). "Varicella and herpes zoster vaccines : WHO position paper, June 2014" (PDF). Weekly Epidemiological Record. 89 (25): 265–287. hdl:10665/242227. PMID 24983077.
- Hall E, Wodi AP, Hamborsky J, Morelli V, Schillie S, eds. (2021). "Chapter 23: Zoster". Epidemiology and Prevention of Vaccine-Preventable Diseases (14th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC).
External links
- Zostavax Product Page U.S. Food and Drug Administration (FDA)
- Shingrix Product Page U.S. Food and Drug Administration (FDA)
- "Shingrix Vaccine Information Statement". U.S. Centers for Disease Control and Prevention (CDC). 29 July 2021.
- "Zostavax (Herpes Zoster Vaccine) Questions and Answers". Questions about Vaccines. U.S. Food and Drug Administration (FDA). 18 February 2021.