Skeletonema costatum
Skeletonema costatum is a cosmopolitan centric diatom that belongs to the genus Skeletonema.[1] It was first described by R. K. Greville, who originally named it Melosira costata, in 1866.[1] It was later renamed by Cleve in 1873 and was more narrowly defined by Zingone et al. and Sarno et al.[2][3][4][5] Skeletonema costatum is the most well known species of the genus Skeletonema and is often one of the dominant species responsible for red tide events.[4][6][7]
| Skeletonema costatum | |
|---|---|
 | |
| Scientific classification | |
| Domain: | Eukaryota |
| Clade: | Diaphoretickes |
| Clade: | SAR |
| Clade: | Stramenopiles |
| Phylum: | Gyrista |
| Subphylum: | Ochrophytina |
| Class: | Bacillariophyceae |
| Order: | Thalassiosirales |
| Family: | Skeletonemataceae |
| Genus: | Skeletonema |
| Species: | S. costatum |
| Binomial name | |
| Skeletonema costatum (Greville) Cleve, 1866 | |
The diatom S. costatum is known for its carbon acquisition mechanisms,[8][9][10][11] and it has been used in the production of biofuel[12][13][14][15] and as a feed for aquaculture.[16][17][18] The organism is appealing for commercial use due to its high photosynthetic efficiency, high tolerance to pH, temperature, and salinity changes, high lipid and fatty acid content, and rapid growth rate.[12][13][14][15][19][20][21][22]
Structure and morphology


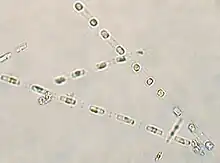
Cells belonging to S. costatum are single-celled but exist as long chains of about 6 to 24 cells[23] but can be up to 60 cells in length.[2] As with all diatoms, the siliceous cell wall (frustule) consists of two interlocking components ("like two halves of a petri dish"[24]), the hypotheca and the epitheca.[25] Each cell is approximately 8 to 12 μm in diameter[5] and about 3.5 to 11.5 μm apart from each other.[23][5] The cells are connected by long straight fultoportula processes[26] and contain up to 2 chloroplasts per cell.[23] Processes are tube-like silicified projections that protrude from the valve wall.[25] Fultoportula processes, also known as strutted processes, protrude through the valve wall with 2 or more satellite pores surrounding them.[24][25] Fultoportula processes are only found in the centric order Thalassiosirales.[24] Each fultoportula process in S. costatum has 3 satellite pores and terminal fultoportulae processes with claw-like tips.[5]
Cells belonging to S. costatum are cylindrically-shaped cells and have a ring of long flattened intercalary fultoportula processes protruding from the periphery of each valve, each closed along their entire length.[2] Each intercalary fultoportula has a longitudinal suture extending from an external pore at its base to its tip.[5]
Intercalary fultoportula processes of adjacent valves are connected at a 1:2 junction, where each process interlocks with two more, creating a "zigzag appearance".[5] This 1:2 junction is a distinctive feature of S. costatum.[5] Their intercalary rimoportula are positioned marginally, they have long terminal rimoportula, and their girdle band features rows of pores.[5] Each valve has one of their fultoportulae replaced with a rimoportula, identified by its longer external process and the "spoutlike teapot"-shaped tip of the terminal rimoportulae.[5]
Morphologically similar species
Among species in the genus Skeletonema , S. costatum is most morphologically similar to S. subsalsum, with both species exhibiting rows of small pores between the parallel rows of transverse branching ribs on their girdle bands.[5] They are also the only two Skeletonema species with long intercalary rimoportula processes.[5] Diatoms in the species S. costatum can be identified by the persistent presence of a 1:2 junction, and the closed tubules of its intercalary fultoportulae processes[5]. Skeletonema subsalsum will sometimes have a 1:1 junction.[5]
Morphological variation
The morphological plasticity of S. costatum cells has been extensively studied.[27][28][29][23] Castillo (1995) attributes significant variations in morphological features, such as cell diameter, number of cells per chain, and the length of intercalary processes, with variations in environmental conditions, most notably, salinity.[23] If cultured in freshwater, S. costatum develops short intercellular processes[30] and is observed to seemingly not have space between sibling valves at 1 psu.[5]
Taxonomy
As of 2021, 21 species in the genus Skeletonema were "identified and taxonomically accepted", with S. costatum being one of them.[31]
Skeletonema costatum was first described by R. K. Greville in 1866 when it was originally called Melosira costata.[1] It was later renamed by Cleve in 1873.[3] Skeletonema costatum has since been more narrowly defined, with numerous species previously attributed to S. costatum identified as distinct species.[2][32][4][5]
The species originally described by Greville is often referred to as S. costatum sensu lato (s. l.),[33][31] which represents multiple different species with similar morphological traits.[5][2][4] The species granted the original epithet, costatum, was the species more narrowly described by Zingone et al. (2005) after reexamination of the type materials of S. costatum using electron microscopy and molecular analysis of rDNA.[2] Zingone et al. (2005) identified two distinct morphologies within the type material, describing the less abundant morphology as S. grevillei and the more abundant morphology as the original epithet, costatum.[2] The latter was assigned the original epithet due to its closer similarity to the specimen originally described by Greville, which he had conveniently marked.[2] The two morphologies differed in their frustule ultrastructures, including "the shape of FPPs [fultoportula processes], the type of interlocking between IFPPs [intercalary fultoportula processes] of sibling valves, and the cingular band ornamentation".[2] S. costatum sensu stricto (s. s.) can be used to describe the more narrowly defined S. costatum species to differentiate it from S. costatum sensu lato.[33] S. costatum sensu stricto (s. s.) has also been referred to as S. costatum (Greville) Cleve emend. Zingone and Sarno.[34][30]
Distribution and habitat
Skeletonema costatum is widely distributed geographically, apart from the Antarctic Ocean.[33] It is found around the world, including off the coasts of Hong Kong Island,[1] Florida, USA, Uruguay, Brazil,[5] Northern Queensland, Australia,[33] China, and the Sea of Japan.[34][26]
Skeletonema costatum primarily resides in the neritic zone and is commonly found in brackish waters as opposed to the more oceanic, S. tropicum.[23] Skeletonema costatum is frequently the dominant phytoplankton species in coastal waters[35][36][37] and the dominant species responsible for red tide events.[6][38][39][40] Although the dominant species in red tide events at a given area can change over time,[6][41] there are studies on red tide events in China in which S. costatum was dominant.[7][39] This include the Yangtze River estuary in the interior of China,[7][42][43] Hongsha Bay of Sanya in the South China Sea,[39] and in Fujian coastal waters.[44][45]
Yangtze River Estuary red tide outbreaks
Skeletonema costatum is one of the dominant species responsible for red tide outbreaks[6] and blooms frequently occur in the Yangtze River estuary and adjacent waters in China.[7][6][43] Of the red tide outbreaks in the Yangtze River estuary between 1972 and 2009, S. costatum occurred during 20% of the 174 recorded outbreaks.[6] Over 50% of the outbreaks in this area occurred during May.[6] After 2000, outbreaks of areal extents larger than 1000 km2 became more common.[6] Nutrient applications from floods, fertilizers and other anthropogenic contributions in this area are suspected to have contributed to the blooms.[7][46]
Growth and environmental conditions
Temperature
Skeletonema costatum grows at a range temperatures from 2 [47] to 31.5 °C,[48] but members of this species grow optimally at 25 °C.[30] The strains of S. costatum from the Sea of Japan off the coast of Dokai Bay prefer warmer temperatures, and are only collected from water above 20 °C.[34] At this temperature, their specific growth rates were measured as above 1.0 d−1.[34]
Salinity
Skeletonema costatum can grow in salinity conditions of 0 to 35 psu.[30] As such, S. costatum can thrive in a variety of ocean environments ranging from oceanic to marine estuary and even riverine environments.[49][50] Its optimal growth was found to be at a salinity range of 18 to 35 psu.[49] The salinity tolerance of S. costatum is especially ideal in estuariane waters where salinities fluctuate, corroborated by the presence of this diatom as one of the dominant species in estuaries.[30]
Although some strains of S. costatum such as SZN B202 exhibit rapid growth rates in salinities of 1 to 2 psu, members of this species generally show decreased growth outside its optimal salinity range.[30] Decreased number of cells in a chain or decreased distance in between cells is observed at stress salinity conditions.[30]
Light levels
Skeletonema costatum is most likely to grow under conditions of high illumination. The highest growth rate was found to be 1.6 x 1016 quanta s−1cm−2, but there is still a positive growth rate in low-light conditions of about 0.02 x 1016 quanta s−1cm−2.[49] There is some debate over the different Skeletonema species and their classification, as many of these different species within the genus bloom in different seasons around the world.[51] S. costatum is a highly adaptable species, and it has the potential to bloom during all seasons.[52][53][54][55] This diatom is more dependent on the water quality than photoperiod lengths for bloom formation, though it is especially common to have a large bloom during the early spring and late summer.[52][53][54][55]
High exposure to UVB radiation can have dramatic effects on the quality of S. costatum as a food source to marine invertebrates, marked by a decrease in fatty acid and amino acid contents of the individual S. costatum cells in these conditions.[56]
The high partial pressures of dissolved CO2 associated with climate change have a positive effect on the growth rate of S. costatum in the spring and fall when there is equal parts light and dark in a day (12h light:12 h dark).[57] The high partial pressure of CO2 was also found to reduce the growth rate of S. costatum in the winter (8h light:16h dark) but had no effect on its growth rate in the summer (16h light:8h dark).[57]
At 20 °C, S. costatum can grow under irradiance of 7 to 406 μmol/m2s.[58] Photoinhibition is observed at 700 μmol/m2s, at which point a decrease in photosynthetic ability of the diatom is detected.[58]
Nutrients
Skeletonema costatum blooms in eutrophic waters that are often loaded with nitrogen, phosphorus, and other nutrients/minerals in both dissolved and particulate forms.[59] The eutrophic conditions that house S. costatum blooms are often limited in carbon dioxide, as there is heavy competition for this limiting resource.[8] It has been shown that nitrate enrichment and waters high in nitrate have the ability to stimulate S. costatum growth through the action of enhanced competitive photosynthetic activity in a CO2-limited environment.[60] Phosphate can also be a limiting nutrient to S. costatum[39] as phosphate-rich waters were found to have a stimulating effect on S. costatum growth rates in CO2-limited environments.[8] High concentrations of nitrates and phosphates increase the amount of inorganic carbon in the form of bicarbonate fixed by S. costatum.[8][60]
Iron is an essential nutrient in primary production as it is used in processes of photosynthesis and is under high competition among marine diatoms. One of the reasons S. costatum is able to outcompete other primary producers is its relatively high uptake rates of iron.[61] S. costatum also has a low cellular demand for iron and is able to obtain this nutrient more efficiently than other phytoplankton.[61]
Major viral pathogens
Skeletonema costatum-infecting virus (ScosV)
Skeletonema costatum-infecting virus (ScosV) is a novel algal virus isolated in 2008 from seawater samples taken in Jaran Bay, South Korea, which infects and lyses specific strains of S. costatum, particularly ME-SCM-1.[62] In 2015, it was characterized as having an icosahedral shape and a diameter of approximately 40 to 50 nm.[62][63] Upon infection of S. costatum, ScosV spends about less than 48 to 80 hours reproducing in the cytoplasm before causing lysis of host cells at a burst size range of 90 to 250 infectious units/cell.[62][63]
Ecological significance
Marine diatoms account for about 20% of the world's primary production[64] and considering that S. costatum is one of the most abundant species blooming in the ocean indicates that it is one of the major producers of oxygen.[65] Skeletonema costatum plays an important role in the acquisition of both organic and inorganic carbon, in the form of HCO3−, in our oceans,[8][9][10][11] collecting CO2 out of the atmosphere and reducing the effects of ocean acidification.[66]
Eutrophic waters, as a result of aquaculture operations in nearshore marine environments such as shrimp farms,[67] create especially favorable conditions for S. costatum growth.[68][69] It has been reported that S. costatum-dominated red tide algal blooms in these eutrophic waters have led to considerable depletion of phosphate that remained at a low level for a long time after the bloom disappeared.[59] These conditions lead to decreased abundance in other phytoplankton species and have potential in impacting the ecosystem in the area where the bloom occurs.[48][70]
It has been suggested that S. costatum may be useful in the remediation of heavy metals from ocean ecosystems as it has a high affinity for iron and other heavy metals like manganese.[59][71]
Human applications
Aquaculture feed
Skeletonema costatum has been cultivated for use in aquaculture as feed for fish, shrimp, oysters, crab larvae, and more.[16][17][18]
Production of biofuels
Skeletonema costatum is used in biofuel production because of its high lipid and fatty acid content,[12][13][14][15][19] rapid growth rate,[21][22] high photosynthetic efficiency,[13][15] and high tolerance to variations in pH, temperature, and salinity levels.[14][20] When exposed to stress conditions such as depleted silicon and phosphate concentrations and high irradiation, it produces neutral lipids like triacylglycerol (TAG) which are ideal for making biofuel.[12] These TAGs are extracted and converted to fatty acid methyl esters (FAME), which are molecules comprising the biofuel, using direct transesterification.[12][13][72]
Production of natural products
Skeletonema costatum is also a source of natural products, which are secondary metabolites produced by microorganisms that can be used in pharmaceutical applications.[73] The extracts of this diatom were found to have prospective use as a central nervous system relaxant that acts like an anti-dopaminergic drug with anticholinergic effects.[74] It also has potential as an antipsychotic drug.[74] Aside from these, antibacterial active compounds extracted from S. costatum using ethanol and methanol were also found to inhibit certain human pathogens, such as Staphylococcus aureus, Proteus mirabilis, and Vibrio cholerae.[73]
References
- GREVILLE, R. K. (1866). "Descriptions of New and Rare Diatoms. Series XVIII". Transactions of the Microscopical Society & Journal. 14 (1): 1–9. doi:10.1111/j.1365-2818.1866.tb05059.x. ISSN 0962-7375. Archived from the original on 19 October 2023. Retrieved 13 March 2022.
- Zingone, Adriana; Percopo, Isabella; Sims, Pat A.; Sarno, Diana (2005). "Diversity in the Genusskeletonema(Bacillariophyceae). I. A Reexamination of the Type Material Ofs. Costatumwith the Description Ofs. Grevilleisp. Nov". Journal of Phycology. 41 (1): 140–150. doi:10.1111/j.1529-8817.2005.04066.x. ISSN 0022-3646. S2CID 84198074. Archived from the original on 19 October 2023. Retrieved 27 March 2022.
- Cleve, P. T. (1873). "Examination of diatoms found on the surface of the sea of Java". Bih. Kongl. Svenska Vetensk.-Akad. Handl. 11: 3–13.
- Sarno, Diana; Kooistra, Wiebe H. C. F.; Medlin, Linda K.; Percopo, Isabella; Zingone, Adriana (February 2005). "Diversity in the Genusskeletonema(Bacillariophyceae). Ii. An Assessment of the Taxonomy Ofs. Costatum-Like Species with the Description of Four New Species". Journal of Phycology. 41 (1): 151–176. doi:10.1111/j.1529-8817.2005.04067.x. S2CID 53679188. Archived from the original on 27 March 2022. Retrieved 27 March 2022.
- Sarno, Diana; Kooistra, Wiebe H. C. F.; Balzano, Sergio; Hargraves, Paul E.; Zingone, Adriana (2007). "Diversity in the Genusskeletonema(Bacillariophyceae): III. Phylogenetic Position and Morphological Variability Ofskeletonema Costatumandskeletonema Grevillei, with the Description Ofskeletonema Ardenssp. Nov". Journal of Phycology. 43 (1): 156–170. doi:10.1111/j.1529-8817.2006.00305.x. ISSN 0022-3646. S2CID 86167912. Archived from the original on 19 October 2023. Retrieved 28 March 2022.
- Liu, Lusan; Zhou, Juan; Zheng, Binghui; Cai, Wenqian; Lin, Kuixuan; Tang, Jingliang (2013). "Temporal and spatial distribution of red tide outbreaks in the Yangtze River Estuary and adjacent waters, China". Marine Pollution Bulletin. 72 (1): 213–221. Bibcode:2013MarPB..72..213L. doi:10.1016/j.marpolbul.2013.04.002. ISSN 0025-326X. PMID 23628547.
- Zhao, Yanfang; Yu, Zhiming; Song, Xiuxian; Cao, Xihua (2009). "Biochemical compositions of two dominant bloom- forming species isolated from the Yangtze River Estuary in response to different nutrient conditions". Journal of Experimental Marine Biology and Ecology. 368 (1): 30–36. doi:10.1016/j.jembe.2008.09.023. ISSN 0022-0981. Archived from the original on 19 October 2023. Retrieved 1 April 2022.
- Gao, Guang; Xia, Jianrong; Yu, Jinlan; Fan, Jiale; Zeng, Xiaopeng (2018). "Regulation of inorganic carbon acquisition in a red tide alga (Skeletonema costatum): the importance of phosphorus availability". Biogeosciences. 15 (16): 4871–4882. Bibcode:2018BGeo...15.4871G. doi:10.5194/bg-15-4871-2018. ISSN 1726-4170. S2CID 203118673. Archived from the original on 31 March 2022. Retrieved 30 March 2022.
- Korb, Rebecca E.; Saville, Peter J.; Johnston, Andrew M.; Raven, John A. (1997). "Sources of Inorganic Carbon for Photosynthesis by Three Species of Marine Diatom1". Journal of Phycology. 33 (3): 433–440. doi:10.1111/j.0022-3646.1997.00433.x. ISSN 0022-3646. S2CID 84794935. Archived from the original on 5 April 2022. Retrieved 5 April 2022.
- Chen, Xiongwen; Gao, Kunshan (2004). "Photosynthetic utilisation of inorganic carbon and its regulation in the marine diatom Skeletonema costatum". Functional Plant Biology. 31 (10): 1027–1033. doi:10.1071/FP04076. ISSN 1445-4416. PMID 32688971. Archived from the original on 25 February 2022. Retrieved 5 April 2022.
- Rost, Björn; Riebesell, Ulf; Sültemeyer, Dieter (2006). "Carbon acquisition of marine phytoplankton: Effect of photoperiod length". Limnology and Oceanography. 51 (1): 12–20. Bibcode:2006LimOc..51...12R. doi:10.4319/lo.2006.51.1.0012. ISSN 0024-3590. S2CID 7827. Archived from the original on 19 October 2023. Retrieved 5 April 2022.
- Popovich, Cecilia A.; Damiani, Cecilia; Constenla, Diana; Leonardi, Patricia I. (1 January 2012). "Lipid quality of the diatoms Skeletonema costatum and Navicula gregaria from the South Atlantic Coast (Argentina): evaluation of its suitability as biodiesel feedstock". Journal of Applied Phycology. 24 (1): 1–10. doi:10.1007/s10811-010-9639-y. hdl:11336/76595. ISSN 1573-5176. S2CID 18983065. Archived from the original on 19 October 2023. Retrieved 1 April 2022.
- Rekha, V.; Gurusamy, R.; Santhanam, P.; Devi, A. Shenbaga; Ananth, S. (April 2012). "Culture and biofuel production efficiency of marine microalgae Chlorella marina and Skeletonema costatum". Indian Journal of Geo-Marine Sciences. 41 (2). ISSN 0975-1033. Archived from the original on 10 September 2019. Retrieved 5 April 2022.
- Dickinson, Selena; Mientus, Miranda; Frey, Daniel; Amini-Hajibashi, Arsalon; Ozturk, Serdar; Shaikh, Faisal; Sengupta, Debalina; El-Halwagi, Mahmoud M. (2017). "A review of biodiesel production from microalgae". Clean Technologies and Environmental Policy. 19 (3): 637–668. doi:10.1007/s10098-016-1309-6. ISSN 1618-9558. S2CID 114548121. Archived from the original on 19 October 2023. Retrieved 5 April 2022.
- Gao, Guang; Wu, Min; Fu, Qianqian; Li, Xinshu; Xu, Juntian (2019). "A two-stage model with nitrogen and silicon limitation enhances lipid productivity and biodiesel features of the marine bloom-forming diatom Skeletonema costatum". Bioresource Technology. 289: 121717. doi:10.1016/j.biortech.2019.121717. ISSN 0960-8524. PMID 31279322. S2CID 195819870.
- Lestari, Dewi Putri; Ekawati, Arning W.; Maftuch, Maftuch (2014). "Dried Skeletonema costatum in Feed Formulation for the Growth of Vaname Shrimp (Litopenaeus vannamei)". The Journal of Experimental Life Sciences. 4 (2): 45–49. doi:10.21776/ub.jels.2014.004.02.04. ISSN 2087-2852. S2CID 86120271.
- Azmi, K. A.; Arsad, S.; Sari, L. A. (2020). "The effect of commercial nutrients to increase the population of Skeletonema costatum on laboratory and mass scales". IOP Conference Series: Earth and Environmental Science. 441 (1): 012039. Bibcode:2020E&ES..441a2039A. doi:10.1088/1755-1315/441/1/012039. ISSN 1755-1315. S2CID 214153049.
- van Houcke, Jasper; Medina, Isabel; Maehre, Hanne K.; Cornet, Josiane; Cardinal, Mireille; Linssen, Jozef; Luten, Joop (2017). "The effect of algae diets (Skeletonema costatum and Rhodomonas baltica) on the biochemical composition and sensory characteristics of Pacific cupped oysters (Crassostrea gigas) during land-based refinement". Food Research International. 100 (Pt 1): 151–160. doi:10.1016/j.foodres.2017.06.041. ISSN 0963-9969. PMID 28873674.
- Xie, Shuyu; Lin, Fan; Zhao, Xin; Gao, Guang (2022). "Enhanced lipid productivity coupled with carbon and nitrogen removal of the diatom Skeletonema costatum cultured in the high CO2 level". Algal Research. 61: 102589. doi:10.1016/j.algal.2021.102589. ISSN 2211-9264. S2CID 245415944.
- Pérez, Leticia; Salgueiro, Jose Luis; González, Jerónimo; Parralejo, Ana Isabel; Maceiras, Rocío; Cancela, Ángeles (2017). "Scaled up from indoor to outdoor cultures of Chaetoceros gracilis and Skeletonema costatum microalgae for biomass and oil production". Biochemical Engineering Journal. 127: 180–187. doi:10.1016/j.bej.2017.08.016. ISSN 1369-703X.
- Doan, Thi Thai Yen; Sivaloganathan, Balasubramanian; Obbard, Jeffrey Philip (2011). "Screening of marine microalgae for biodiesel feedstock". Biomass and Bioenergy. 35 (7): 2534–2544. doi:10.1016/j.biombioe.2011.02.021. ISSN 0961-9534.
- Sharmin, Tania; Monirul Hasan, Chowdhury Md; Aftabuddin, Sheikh; Rahman, Md Atiar; Khan, Mala (2016). "Growth, Fatty Acid, and Lipid Composition of Marine Microalgae Skeletonema costatum Available in Bangladesh Coast: Consideration as Biodiesel Feedstock". Journal of Marine Biology. 2016: e6832847. doi:10.1155/2016/6832847. ISSN 2633-4666.
- Castillo, J. Aké; Meave del Castillo, M.E.; Hernández-Becerril, D.U. (1995). "Morphology and distribution of species of the diatom genus Skeletonema in a tropical coastal lagoon". European Journal of Phycology. 30 (2): 107–115. doi:10.1080/09670269500650871. ISSN 0967-0262.
- Round, F. E.; Crawford, R. M.; Mann, D. G. (1990). Diatoms: Biology and Morphology of the Genera. Cambridge University Press. ISBN 978-0-521-36318-1. Archived from the original on 19 October 2023. Retrieved 6 April 2022.
- Ross, R.; Cox, Eileen; Karayeva, N.I.; Mann, David; Paddock, T.B.B.; Simonsen, R.; Sims, Pat (1979). "An amended terminology for the siliceous components of the Diatom cell". Nova Hedwigia, Beihefte. 64: 513–533.
- Shevchenko, Olga G.; Ponomareva, Anna A.; Shulgina, Maria A.; Orlova, Tatiana Yu. (2019). "Phytoplankton in the Coastal Waters of Russky Island, Peter the Great Bay, Sea of Japan". Botanica Pacifica. doi:10.17581/bp.2019.08112. S2CID 135142997.
- Castellví, Josefina (1969). "Long polar fibres of Skeletonema costatum (Grev.) Cleve". Proc. Int. Seaweed Symp. 6: 85–88. hdl:10261/166850. Archived from the original on 19 October 2023. Retrieved 27 March 2022.
- Hasle, G. R. (1973). "Morphology and taxonomy of Skeletonema costatum (Bacillariophyceae)". Norwegian Journal of Botany. 20: 109–137.
- Gallagher, Jane C. (1982). "Physiological Variation and Electrophoretic Banding Patterns of Genetically Different Seasonal Populations of Skeletonema Costatum (Bacillariophyceae)". Journal of Phycology. 18 (1): 148–162. doi:10.1111/j.1529-8817.1982.tb03169.x. ISSN 0022-3646. S2CID 86231102. Archived from the original on 19 October 2023. Retrieved 27 March 2022.
- Balzano, S.; Sarno, D.; Kooistra, W. H. C. F. (2011). "Effects of salinity on the growth rate and morphology of ten Skeletonema strains". Journal of Plankton Research. 33 (6): 937–945. doi:10.1093/plankt/fbq150. ISSN 0142-7873.
- Liu, Shuya; Wang, Yichao; Xu, Qing; Zhang, Mengjia; Chen, Nansheng (2021). "Comparative analysis of full-length mitochondrial genomes of five Skeletonema species reveals conserved genome organization and recent speciation". BMC Genomics. 22 (1): 746. doi:10.1186/s12864-021-07999-z. ISSN 1471-2164. PMC 8520197. PMID 34654361.
- Medlin, Linda K.; Elwood, Hille J.; Stickel, Shawn; Sogin, Mitchell L. (1991). "Morphological and Genetic Variation within the Diatom Skeletonema Costatum (Bacillariophyta): Evidence for a New Species, Skeletonema Pseudocostatum1". Journal of Phycology. 27 (4): 514–524. doi:10.1111/j.0022-3646.1991.00514.x. ISSN 0022-3646. S2CID 84971128. Archived from the original on 19 October 2023. Retrieved 31 March 2022.
- Kooistra, Wiebe H.C.F.; Sarno, Diana; Balzano, Sergio; Gu, Haifeng; Andersen, Robert A.; Zingone, Adriana (2008). "Global Diversity and Biogeography of Skeletonema Species (Bacillariophyta)". Protist. 159 (2): 177–193. doi:10.1016/j.protis.2007.09.004. PMID 18042429. Archived from the original on 3 March 2022. Retrieved 28 March 2022.
- Yamada, Machiko; Katsuki, Eri; Otsubo, Mayuko; Kawaguchi, Mayumi; Ichimi, Kazuhiko; Kaeriyama, Hideki; Tada, Kuninao; Harrison, Paul J. (2010). "Species diversity of the genus Skeletonema (Bacillariophyceae) in the industrial harbor Dokai Bay, Japan". Japan Journal of Oceanography. 66 (6): 755–771. doi:10.1007/s10872-010-0062-4. ISSN 0916-8370. S2CID 85043336. Archived from the original on 19 October 2023. Retrieved 28 March 2022.
- Balkis, N. (2003). "Seasonal variations in the phytoplankton and nutrient dynamics in the neritic water of Buyukcekmece Bay, Sea of Marmara". Journal of Plankton Research. 25 (7): 703–717. doi:10.1093/plankt/25.7.703. ISSN 1464-3774. Archived from the original on 19 October 2023. Retrieved 8 April 2022.
- Marshall, Harold G.; Ananda Ranasinghe, J. (1989). "Phytoplankton distribution along the eastern coast of the U.S.A.—VII. Mean cell concentrations and standing crop". Continental Shelf Research. 9 (2): 153–164. Bibcode:1989CSR.....9..153M. doi:10.1016/0278-4343(89)90089-7. ISSN 0278-4343. Archived from the original on 19 October 2023. Retrieved 8 April 2022.
- Ramaiah, N; Furuya, K (2002). "Seasonal variations in phytoplankton composition and transparent exopolymer particles in a eutrophicated coastal environment". Aquatic Microbial Ecology. 30: 69–82. doi:10.3354/ame030069. ISSN 0948-3055.
- Baohong, Chen; Kang, Wang; Xu, Dong; Hui, Lin (2021). "Long-term changes in red tide outbreaks in Xiamen Bay in China from 1986 to 2017". Estuarine, Coastal and Shelf Science. 249: 107095. Bibcode:2021ECSS..24907095B. doi:10.1016/j.ecss.2020.107095. ISSN 0272-7714. S2CID 229476076. Archived from the original on 19 October 2023. Retrieved 28 March 2022.
- Li, Chunqiang; Zhu, Baibi; Chen, Hong; Liu, Zhixin; Cui, Baiming; Wu, Jingrui; Li, Bin; Yu, Haichuan; Peng, Ming (2009). "The Relationship between the Skeletonema costatum Red Tide and Environmental Factors in Hongsha Bay of Sanya, South China Sea". Journal of Coastal Research. 253: 651–658. doi:10.2112/07-0967.1. ISSN 0749-0208. S2CID 88660510. Archived from the original on 19 October 2023. Retrieved 28 March 2022.
- Hilaluddin, Fareha; Yusoff, Fatimah Md.; Toda, Tatsuki (2020). "Shifts in Diatom Dominance Associated with Seasonal Changes in an Estuarine-Mangrove Phytoplankton Community". Journal of Marine Science and Engineering. 8 (7): 528. doi:10.3390/jmse8070528. ISSN 2077-1312.
- ZHANG, Fei-Yan; TANG, Jing-Liang; LI, Dao-Ji; FANG, Tao; WANG, Biao (2007). "Zooplankton Distribution and Variation in the Yangtze Estuary and ITS Adjacent Waters in Summer and Autumn". Acta Hydrobiologica Sinica. 33 (6): 1219–1225. doi:10.3724/sp.j.1035.2009.61219. ISSN 1000-3207.
- SHI, Yunrong; CHAO, Min; QUAN, Weimin; TANG, Fenghua; SHEN, Xinqiang; YUAN, Qi; HUANG, Houjian (3 September 2013). "Spatial variation in fish community of Yangtze River estuary in spring". Journal of Fishery Sciences of China. 18 (5): 1141–1151. doi:10.3724/sp.j.1118.2011.01141. ISSN 1005-8737.
- Zhu, Zhuo-Yi; Ng, Wai-Man; Liu, Su-Mei; Zhang, Jing; Chen, Jay-Chung; Wu, Ying (2009). "Estuarine phytoplankton dynamics and shift of limiting factors: A study in the Changjiang (Yangtze River) Estuary and adjacent area". Estuarine, Coastal and Shelf Science. 84 (3): 393–401. Bibcode:2009ECSS...84..393Z. doi:10.1016/j.ecss.2009.07.005. ISSN 0272-7714. Archived from the original on 19 October 2023. Retrieved 1 April 2022.
- Li, Bing-Han; Liu, Chun-Ying; Deng, Xue; Wang, Ke-Ke; Han, Lu; Huang, Yu-Huan; Li, Xinyu; Cai, Wei-Jun (December 2021). "Responses of the marine carbonate system to a green tide: A case study of an Ulva prolifera bloom in Qingdao coastal waters". Harmful Algae. 110: 102133. doi:10.1016/j.hal.2021.102133. ISSN 1568-9883. PMID 34887011. S2CID 244453233. Archived from the original on 19 October 2023. Retrieved 8 April 2022.
- Baohong, Chen; Kang, Wang; Xu, Dong; Hui, Lin (February 2021). "Long-term changes in red tide outbreaks in Xiamen Bay in China from 1986 to 2017". Estuarine, Coastal and Shelf Science. 249: 107095. Bibcode:2021ECSS..24907095B. doi:10.1016/j.ecss.2020.107095. ISSN 0272-7714. S2CID 229476076. Archived from the original on 19 October 2023. Retrieved 28 March 2022.
- Li, Maotian; Xu, Kaiqin; Watanabe, Masataka; Chen, Zhongyuan (1 January 2007). "Long-term variations in dissolved silicate, nitrogen, and phosphorus flux from the Yangtze River into the East China Sea and impacts on estuarine ecosystem". Estuarine, Coastal and Shelf Science. Sedimentological and ecohydrological processes of Asian deltas: The Yangtze and the Mekong. 71 (1): 3–12. Bibcode:2007ECSS...71....3L. doi:10.1016/j.ecss.2006.08.013. ISSN 0272-7714.
- Hitchcock, G. L. (1 October 1980). "Influence of temperature on the growth rate of Skeletonema costatum in response to variations in daily light intensity". Marine Biology. 57 (4): 261–269. doi:10.1007/BF00387569. ISSN 1432-1793. S2CID 86752075. Archived from the original on 19 October 2023. Retrieved 1 April 2022.
- Redzuan, Nurul & Milow, Pozi. (2019). Skeletonema costatum of mangrove ecosystem: Its dynamics across physico-chemical parameters variability. AACL Bioflux. 12. 179-190.
- Tian, Yan; Mingjiang, Zhou; Peiyuan, Qian (1 September 2002). "Combined effects of temperature, irradiance and salinity on growth of diatomSkeletonema costatum". Chinese Journal of Oceanology and Limnology. 20 (3): 237–243. Bibcode:2002ChJOL..20..237Y. doi:10.1007/BF02848852. ISSN 1993-5005. S2CID 89815663. Archived from the original on 19 October 2023. Retrieved 30 March 2022.
- Castillo, J. Aké; Meave del Castillo, M.E.; Hernández-Becerril, D.U. (1 May 1995). "Morphology and distribution of species of the diatom genus Skeletonema in a tropical coastal lagoon". European Journal of Phycology. 30 (2): 107–115. doi:10.1080/09670269500650871. ISSN 0967-0262.
- Assmy, P.; Smetacek, V. (1 January 2009), "Algal Blooms", in Schaechter, Moselio (ed.), Encyclopedia of Microbiology (Third Edition), Oxford: Academic Press, pp. 27–41, doi:10.1016/b978-012373944-5.00001-8, ISBN 978-0-12-373944-5, archived from the original on 1 April 2022, retrieved 1 April 2022
- Canesi, Kl; Rynearson, Ta (8 September 2016). "Temporal variation of Skeletonema community composition from a long-term time series in Narragansett Bay identified using high-throughput DNA sequencing". Marine Ecology Progress Series. 556: 1–16. Bibcode:2016MEPS..556....1C. doi:10.3354/meps11843. ISSN 0171-8630. Archived from the original on 1 April 2022. Retrieved 1 April 2022.
- Álvarez–Salgado, X. A.; Nieto–Cid, M.; Piedracoba, S.; Crespo, B. G.; Gago, J.; Brea, S.; Teixeira, I. G.; Figueiras, F. G.; Garrido, J. L.; Rosón, G.; Castro, C. G. (1 November 2005). "Origin and fate of a bloom of Skeletonema costatum during a winter upwelling/downwelling sequence in the Ría de Vigo (NW Spain)". Journal of Marine Research. 63 (6): 1127–1149. doi:10.1357/002224005775247616. hdl:10261/55844.
- Sun, Bai-Ye; Liang, Sheng-Kang; Wang, Chang-You; Wang, Xiao-Bo; Wang, Xiu-Lin; Li, Yan-Bin (July 2008). "[Role of irradiance on the seasonality of Skeletonema costatum Cleve blooms in the coastal area in East China Sea]". Huan Jing Ke Xue = Huanjing Kexue. 29 (7): 1849–1854. ISSN 0250-3301. PMID 18828365. Archived from the original on 1 April 2022. Retrieved 1 April 2022.
- Han, Myung-Soo; Furuya, Ken; Nemoto, Takahisa (1992). "Species-specific productivity of Skeletonema costatum (Bacillariophyceae) in the inner part of Tokyo Bay". Marine Ecology Progress Series. 79 (3): 267–273. doi:10.3354/meps079267. ISSN 0171-8630. JSTOR 44634807.
- Nahon, Sarah; Charles, François; Lantoine, François; Vétion, Gilles; Escoubeyrou, Karine; Desmalades, Martin; Pruski, Audrey M. (15 February 2010). "Ultraviolet radiation negatively affects growth and food quality of the pelagic diatom Skeletonema costatum". Journal of Experimental Marine Biology and Ecology. 383 (2): 164–170. doi:10.1016/j.jembe.2009.12.006. ISSN 0022-0981.
- Li, Hangxiao; Xu, Tianpeng; Ma, Jing; Li, Futian; Xu, Juntian (24 February 2021). "Physiological responses of <i>Skeletonema costatum</i> to the interactions of seawater acidification and the combination of photoperiod and temperature". Biogeosciences. 18 (4): 1439–1449. Bibcode:2021BGeo...18.1439L. doi:10.5194/bg-18-1439-2021. ISSN 1726-4189. S2CID 233993815. Archived from the original on 1 April 2022. Retrieved 1 April 2022.
- Kaeriyama, Hideki; Katsuki, Eri; Otsubo, Mayuko; Yamada, Machiko; Ichimi, Kazuhiko; Tada, Kuninao; Harrison, Paul J. (1 May 2011). "Effects of temperature and irradiance on growth of strains belonging to seven Skeletonema species isolated from Dokai Bay, southern Japan". European Journal of Phycology. 46 (2): 113–124. doi:10.1080/09670262.2011.565128. ISSN 0967-0262. S2CID 85648271.
- Huo, Wen Yi; Shu, Jian Jun (2005). "Outbreak of skeletonema costatum bloom and its relations to environmental factors in Jiaozhou Bay, China". Archived from the original on 14 May 2022. Retrieved 29 March 2022.
{{cite journal}}: Cite journal requires|journal=(help) - Gao, Guang; Xia, Jianrong; Yu, Jinlan; Zeng, Xiaopeng (1 February 2018). "Physiological response of a red tide alga (Skeletonema costatum) to nitrate enrichment, with special reference to inorganic carbon acquisition". Marine Environmental Research. 133: 15–23. Bibcode:2018MarER.133...15G. doi:10.1016/j.marenvres.2017.11.003. ISSN 0141-1136. PMID 29174425.
- Wang, Bo; Chen, Min; Zheng, Minfang; Qiu, Yusheng (26 February 2020). "Responses of Two Coastal Algae (Skeletonema costatumandChlorella vulgaris) to Changes in Light and Iron Levels1". Journal of Phycology. 56 (3): 618–629. doi:10.1111/jpy.12972. ISSN 0022-3646. PMID 31965566. S2CID 210862256. Archived from the original on 19 October 2023. Retrieved 1 April 2022.
- Kim, JinJoo; Kim, Chang-Hoon; Youn, Seok-Hyun; Choi, Tae-Jin (June 2015). "Isolation and Physiological Characterization of a Novel Algicidal Virus Infecting the Marine Diatom Skeletonema costatum". The Plant Pathology Journal. 31 (2): 186–191. doi:10.5423/PPJ.NT.03.2015.0029. ISSN 1598-2254. PMC 4454000. PMID 26060438.
- Short, Steven M.; Staniewski, Michael A.; Chaban, Yuri V.; Long, Andrew M.; Wang, Donglin (2020). "Diversity of Viruses Infecting Eukaryotic Algae". Current Issues in Molecular Biology. 39: 29–62. doi:10.21775/cimb.039.029. PMID 32073403. S2CID 211193770. Archived from the original on 1 April 2022. Retrieved 1 April 2022.
- Field, Christopher B.; Behrenfeld, Michael J.; Randerson, James T.; Falkowski, Paul (10 July 1998). "Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components". Science. 281 (5374): 237–240. Bibcode:1998Sci...281..237F. doi:10.1126/science.281.5374.237. ISSN 0036-8075. PMID 9657713. Archived from the original on 19 October 2023. Retrieved 29 March 2022.
- Degerlund, Maria; Eilertsen, Hans Christian (March 2010). "Main Species Characteristics of Phytoplankton Spring Blooms in NE Atlantic and Arctic Waters (68–80° N)". Estuaries and Coasts. 33 (2): 242–269. doi:10.1007/s12237-009-9167-7. ISSN 1559-2723. S2CID 84299059. Archived from the original on 19 October 2023. Retrieved 1 April 2022.
- Sethi, Deepak; Butler, Thomas O.; Shuhaili, Faqih; Vaidyanathan, Seetharaman (10 August 2020). "Diatoms for Carbon Sequestration and Bio-Based Manufacturing". Biology. 9 (8): 217. doi:10.3390/biology9080217. ISSN 2079-7737. PMC 7464044. PMID 32785088.
- Herbeck, Lucia S.; Unger, Daniela; Wu, Ying; Jennerjahn, Tim C. (1 April 2013). "Effluent, nutrient and organic matter export from shrimp and fish ponds causing eutrophication in coastal and back-reef waters of NE Hainan, tropical China". Continental Shelf Research. Land – Sea Interactions in Tropical Ecosystems of Hainan, China. 57: 92–104. Bibcode:2013CSR....57...92H. doi:10.1016/j.csr.2012.05.006. ISSN 0278-4343. Archived from the original on 2 April 2017. Retrieved 1 April 2022.
- Qiu, Dajun; Huang, Liangmin; Zhang, Jianlin; Lin, Senjie (15 February 2010). "Phytoplankton dynamics in and near the highly eutrophic Pearl River Estuary, South China Sea". Continental Shelf Research. 30 (2): 177–186. Bibcode:2010CSR....30..177Q. doi:10.1016/j.csr.2009.10.015. ISSN 0278-4343.
- Nóbrega, G. N.; Otero, X. L.; Macías, F.; Ferreira, T. O. (September 2014). "Phosphorus geochemistry in a Brazilian semiarid mangrove soil affected by shrimp farm effluents". Environmental Monitoring and Assessment. 186 (9): 5749–5762. doi:10.1007/s10661-014-3817-3. ISSN 0167-6369. PMID 24838803. S2CID 25249231. Archived from the original on 19 October 2023. Retrieved 1 April 2022.
- Li, Chunqiang; Zhu, Baibi; Chen, Hong; Liu, Zhixin; Cui, Baiming; Wu, Jingrui; Li, Bin; Yu, Haichuan; Peng, Ming (May 2009). "The Relationship between the Skeletonema costatum Red Tide and Environmental Factors in Hongsha Bay of Sanya, South China Sea". Journal of Coastal Research. 253: 651–658. doi:10.2112/07-0967.1. ISSN 0749-0208. S2CID 88660510. Archived from the original on 19 October 2023. Retrieved 6 April 2022.
- K, Kranthi Raj; Sardar, Usha R.; Bhargavi, Erravelli; Devi, Indrama; Bhunia, Biswanath; Tiwari, Onkar Nath (1 November 2018). "Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: A critical review". Carbohydrate Polymers. 199: 353–364. doi:10.1016/j.carbpol.2018.07.037. ISSN 0144-8617. PMID 30143139. S2CID 52079031.
- Lee, Adam F.; Bennett, James A.; Manayil, Jinesh C.; Wilson, Karen (20 October 2014). "Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification". Chemical Society Reviews. 43 (22): 7887–7916. doi:10.1039/C4CS00189C. ISSN 1460-4744. PMID 24957179. S2CID 15059976.
- Naviner, M; Bergé, J.-P; Durand, P; Le Bris, H (1999). "Antibacterial activity of the marine diatom Skeletonema costatum against aquacultural pathogens". Aquaculture. 174 (1–2): 15–24. doi:10.1016/s0044-8486(98)00513-4. ISSN 0044-8486. Archived from the original on 19 October 2023. Retrieved 6 April 2022.
- Villar, R.; Laguna, M.; Calleja, J.; Cadavid, I. (October 1992). "Effects of Skeletonema costatum Extracts on the Central Nervous System". Planta Medica. 58 (5): 398–404. doi:10.1055/s-2006-961500. ISSN 0032-0943. PMID 1470662. S2CID 32043623. Archived from the original on 1 June 2018. Retrieved 6 April 2022.