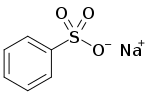
Sodium benzenesulfonate
Sodium benzenesulfonate is an organic compound with the formula C6H5SO3Na. It is white, water-soluble solid, It is produced by the neutralization benzenesulfonic acid with sodium hydroxide. It is also a common ingredient in some detergents. The compound typically crystallizes from water as the monohydrate.[1]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.454 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H5NaO3S | |
| Molar mass | 180.15 g·mol−1 |
| Appearance | white solid |
| Melting point | 450 °C (842 °F; 723 K) decomposition |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Heating this salt in strong base results in desulfonation, giving, after acid workup, phenol[2] This reaction was at one time, the principal route to phenol.
References
- Otto Lindner; Lars Rodefeld (2005). "Benzenesulfonic Acids and Their Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_507.
- W. W. Hartman (1923). "p-Cresol". Organic Syntheses. 3: 37. doi:10.15227/orgsyn.003.0037.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.