Sodium arsenite
Sodium arsenite usually refers to the inorganic compound with the formula NaAsO2. Also called sodium meta-arsenite, it is the sodium salt of arsenous acid. Sodium ortho-arsenite is Na3AsO3.[2] The compounds are colourless solids.
 | |
| Names | |
|---|---|
| IUPAC name
sodium arsenite | |
| Other names
sodium arsenate(III) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.029.154 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1686 2027 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| NaAsO2 | |
| Molar mass | 129.91 g/mol |
| Appearance | white or grayish powder hygroscopic |
| Density | 1.87 g/cm 3 |
| Melting point | 550 °C (1,022 °F; 823 K) decomposes |
| 156 g/100 mL | |
| Solubility | slightly soluble in alcohol |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H300, H301, H310, H331, H350, H410 | |
| P201, P202, P261, P262, P264, P270, P271, P273, P280, P281, P301+P310, P302+P350, P302+P352, P304+P340, P308+P313, P310, P311, P312, P321, P322, P330, P361, P363, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
41 mg/kg (rat, oral) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
[1910.1018] TWA 0.010 mg/m3[1] |
REL (Recommended) |
Ca C 0.002 mg/m3 [15-minute][1] |
IDLH (Immediate danger) |
Ca [5 mg/m3 (as As)][1] |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
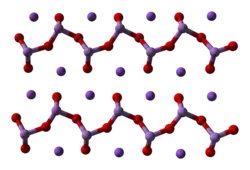
Synthesis and structure
A mixture of sodium meta-arsenite and sodium ortho-arsenite is produced by treating arsenic trioxide with sodium carbonate or sodium hydroxide.[3] Sodium arsenite is amorphous, typically being obtained as a powder or as a glassy mass. The compound consists of the polymer [AsO2]n−
n associated with sodium cations, Na+. The polymer backbone has the connectivity -O-As(O−)-.[4]
Health effects
Sodium arsenite can be inhaled or absorbed through the skin. Along with its known carcinogenic and teratogenic effects, contact with the substance can yield symptoms such as skin irritation, burns, itching, thickened skin, rash, loss of pigment, poor appetite, a metallic or garlic taste, stomach pain, nausea, vomiting, diarrhea, convulsions, decreased blood pressure, and headache. Severe acute poisoning may lead to nervous system damage resulting in weakness, poor coordination, or “pins and needles” sensations, eventual paralysis, and death.[5][6]
Application
It is primarily used as a pesticide, but has other uses such as hide preservative, antiseptic, dyeing, and soaps.[7]
Sodium arsenite is an appropriate chemical stressor to induce the production of heat shock proteins,[8] and the formation of cytoplasmic stress granules.[9]
Sodium arsenite can be used as a reducing agent in organic chemistry, as it is able to reduce a trihaloalkane to a dihaloalkane:
- CHBr3 + Na3AsO3 + NaOH → CH2Br2 + Na3AsO4 + NaBr
Safety
The LD50 (oral, mouse) is 40 mg/kg.[3]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0038". National Institute for Occupational Safety and Health (NIOSH).
- Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- Grund, S. C.; Hanusch, K.; Wolf, H. U. "Arsenic and Arsenic Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_113.pub2.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - Eagleton M. (2011). Concise Encyclopedia Chemistry. Walter de Gruyter. ISBN 978-3110114515.
- New Jersey Department of Health and Senior Services. Hazardous Substance Fact Sheet: Sodium Arsenite (2013-05-01)
- Jing J; Zheng G; Liu M; Shen X; Zhao F; Wang J; Zhang J; Huang G; Dai P; Chen Y; Chen J; Luo W; ‘’et al.’’ (2012). "Changes in the synaptic structure of hippocampal neurons and impairment of spatial memory in a rat model caused by chronic arsenite exposure". Neurotoxicology. 33 (5): 1230–8. doi:10.1016/j.neuro.2012.07.003. PMID 22824511.
- Considine G.D. (2005). Van Nostrand's Encyclopedia of Chemistry. 14th Ed. Wiley. ISBN 0471615250.
- Bhagat L; Singh VP; Dawra RK; Saluja AK; ‘’et al.’’ (2008). "Sodium arsenite induces heat shock protein 70 expression and protects against secretagogue-induced trypsinogen and NF-kappaB activation". J Cell Physiol. 215 (1): 37–46. doi:10.1002/jcp.21286. PMID 17941083. S2CID 13325879.
- McEwen, Edward; Kedersha, Nancy; Song, Benbo; Scheuner, Donalyn; Gilks, Natalie; Han, Anping; Chen, Jane-Jane; Anderson, Paul; Kaufman, Randal J. (2005-04-29). "Heme-regulated Inhibitor Kinase-mediated Phosphorylation of Eukaryotic Translation Initiation Factor 2 Inhibits Translation, Induces Stress Granule Formation, and Mediates Survival upon Arsenite Exposure". Journal of Biological Chemistry. 280 (17): 16925–16933. doi:10.1074/jbc.M412882200. ISSN 0021-9258. PMID 15684421.
