Sodium methylsulfinylmethylide
Sodium methylsulfinylmethylide (also called NaDMSO or dimsyl sodium) is the sodium salt of the conjugate base of dimethyl sulfoxide. This unusual salt has some uses in organic chemistry as a base and nucleophile.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium (methanesulfinyl)methanide | |
| Other names
sodium dimsylate, dimsylsodium, NaDMSYL | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | NaDMSO |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H5NaOS | |
| Molar mass | 100.13 |
| Appearance | White solid, solution in DMSO is green |
| Reacts forming DMSO | |
| Solubility | Very soluble in DMSO and many polar organic solvents |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
decomposes to corrosive NaOH, May be explosive in certain circumstances [1] |
| Related compounds | |
Related compounds |
Dimethyloxosulfonium methylide, dimethyl sulfoxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Since the first publication in 1965 by Corey et al.,[2] a number of additional uses for this reagent have been identified.[3]
Preparation
Sodium methylsulfinylmethylide is prepared by heating sodium hydride[4] or sodium amide[5] in DMSO[6]
- CH3SOCH3 + NaH → CH3SOCH2−Na+ + H2
- CH3SOCH3 + NaNH2 → CH3SOCH2−Na+ + NH3
Reactions
As a Base
The pKa of DMSO is 35, which leads NaDMSO to be a powerful Brønsted base. NaDMSO is used in the generation of phosphorus and sulfur ylides.[7] NaDMSO in DMSO is especially convenient in the generation of dimethyloxosulfonium methylide and dimethylsulfonium methylide.[2][8]
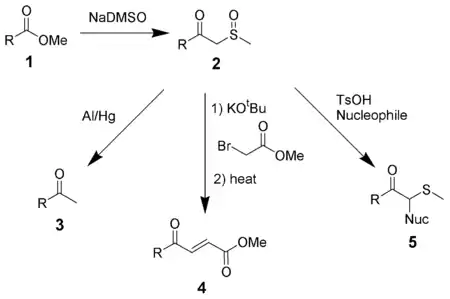
Reaction with esters
NaDMSO condenses with esters (1) to form β-ketosulfoxides (2), which can be useful intermediates.[9] Reduction of β-ketosulfoxides with aluminium amalgam gives methyl ketones (3).[10] Reaction with alkyl halides followed by elimination gives α,β-unsaturated ketones (4). β-ketosulfoxides can also be used in the Pummerer rearrangement to introduce nucleophiles alpha to a carbonyl (5).[11]
References
- "Sodium Hydride in Aprotic Solvents: Look Out".
- Corey, E. J.; Chaykovsky, M. (1965). "Methylsulfinyl Carbanion (CH3-SO-CH2−). Formation and Applications to Organic Synthesis". J. Am. Chem. Soc. 87 (6): 1345–1353. doi:10.1021/ja01084a033.
- Mukulesh Mondal "Sodium methylsulfinylmethylide: A versatile reagent" Synlett 2005, vol. 17, 2697-2698. doi:10.1055/s-2005-917075
- Iwai, I.; Ide, J. (1988). "2,3-Diphenyl-1,3-Butadiene". Organic Syntheses.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 6, p. 531 - Kaiser, E. M.; Beard, R. D.; Hauser, C. R. (1973). "Preparation and reactions of the mono- and dialkali salts of dimethyl sulfone, dimethyl sulfoxide, and related compounds". J. Organomet. Chem. 59: 53–64. doi:10.1016/S0022-328X(00)95020-4.
- "Preparation of dimsyl sodium".
- Romo, D.; Myers, A. I. (1992). "An asymmetric route to enantiomerically pure 1,2,3-trisubstituted cyclopropanes". J. Org. Chem. 57 (23): 6265–6270. doi:10.1021/jo00049a038.
- Trost, B. M.; Melvin, L. S., Jr. (1975). Sulfur Ylides: Emerging Synthetic Intermediates. New York: Academic Press. ISBN 0-12-701060-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Ibarra, C. A; Rodgríguez, R. C; Monreal, M. C. F; Navarro, F. J. G.; Tesoreo, J. M. (1989). "One-pot synthesis of β-keto sulfones and β-keto sulfoxides from carboxylic acids". J. Org. Chem. 54 (23): 5620–5623. doi:10.1021/jo00284a043.
- Swenton, J. S.; Anderson, D. K.; Jackson, D. K.; Narasimhan, L. (1981). "1,4-Dipole-metalated quinone strategy to (±)-4-demethoxydaunomycinone and (±)-daunomycinone. Annelation of benzocyclobutenedione monoketals with lithioquinone bisketals". J. Org. Chem. 46 (24): 4825–4836. doi:10.1021/jo00337a002.
- Isibashi, H.; Okada, M.; Komatsu, H.; Ikeda, M. S. (1985). "A New Synthesis of Substituted Cyclopentenones by Olefin Cyclization Initiated by Pummerer Reaction Intermediates". Synthesis. 1985 (6/7): 643–645. doi:10.1055/s-1985-31290. S2CID 95643470.
External links
- "The Dimethyl Sulfoxide (DMSO) Anion — Dimsyl Ion" (PDF). Gaylord Chemical Corporation. October 2007. Archived from the original (PDF) on 2011-07-11.
- "Preparation of dimsyl sodium". June 2009.