Soticlestat
Soticlestat (TAK-935, OV-935) is an experimental anticonvulsant and cholesterol 24-hydroxylase inhibitor being investigated as a treatment for Dravet syndrome, Lennox–Gastaut syndrome, tuberous sclerosis complex, dup15q syndrome, and CDKL5 deficiency disorder.[1][2] The development rights to the drug were purchased by Takeda Pharmaceuticals from Ovid Therapeutics in 2021.[3]
 | |
| Clinical data | |
|---|---|
| Other names | TAK-935; OV-935 |
| Routes of administration | By mouth |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
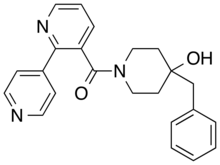
| Formula | C23H23N3O2 |
| Molar mass | 373.456 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Soticlestat was designated as an orphan drug by the FDA in 2017 for the treatment for both Dravet syndrome and Lennox–Gastaut syndrome.[4] In a phase 2 study called ELEKTRA, soticlestat was well tolerated and reduced seizure frequency in patients with Dravet syndrome and Lennox–Gastaut syndrome.[5]
Mechanism of action
Soticlestat functions by blocking cholesterol 24-hydroxylase (CH24H, also known as CYP46A1), an enzyme in the brain that converts cholesterol to the oxysterol 24S-hydroxycholesterol (24S-HC). Reduction of 24S-HC has been shown to reduce glutamatergic signaling, which reduces seizures.[1][2] Soticlestat may also have neuroprotective and anti-inflammatory properties via glial cell modulation.[2]
References
- Hong W, Haviland I, Pestana-Knight E, Weisenberg JL, Demarest S, Marsh ED, Olson HE (June 2022). "CDKL5 Deficiency Disorder-Related Epilepsy: A Review of Current and Emerging Treatment". CNS Drugs. 36 (6): 591–604. doi:10.1007/s40263-022-00921-5. PMC 9876658. PMID 35633486.
- Pong AW, Ross J, Tyrlikova I, Giermek AJ, Kohli MP, Khan YA, Salgado RD, Klein P (March 2022). "Epilepsy: expert opinion on emerging drugs in phase 2/3 clinical trials". Expert Opinion on Emerging Drugs. 27 (1): 75–90. doi:10.1080/14728214.2022.2059464. PMID 35341431. S2CID 247763576.
- "Takeda buys epilepsy treatment rights from Ovid for up to $856 million". Reuters. 3 March 2021. Retrieved 2 October 2023.
- Pena, Ana (22 October 2019). "Soticlestat Can Reduce Seizure Frequency in Adults With Dravet, Other Rare Epilepsies, Initial Data Shows". Dravet Syndrome News. Retrieved 2 October 2023.
- Hahn CD, Jiang Y, Villanueva V, Zolnowska M, Arkilo D, Hsiao S, Asgharnejad M, Dlugos D (October 2022). "A phase 2, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of soticlestat as adjunctive therapy in pediatric patients with Dravet syndrome or Lennox–Gastaut syndrome (ELEKTRA)". Epilepsia. 63 (10): 2671–2683. doi:10.1111/epi.17367. PMC 9804149. PMID 35841234.