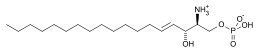
Sphingosine-1-phosphate
Sphingosine-1-phosphate (S1P) is a signaling sphingolipid, also known as lysosphingolipid. It is also referred to as a bioactive lipid mediator. Sphingolipids at large form a class of lipids characterized by a particular aliphatic aminoalcohol, which is sphingosine.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2S,3R,4E)-2-Amino-3-hydroxyoctadec-4-en-1-yl dihydrogen phosphate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.164.436 |
| KEGG | |
| MeSH | sphingosine+1-phosphate |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H38NO5P | |
| Molar mass | 379.472 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Production
S1P is formed from ceramide,[1] which is composed of a sphingosine and a fatty acid. Ceramidase, an enzyme primarily present in plasma membrane, will convert ceramide to sphingosine.[1] sphingosine is then phosphorylated by sphingosine kinase (SK) isoenzymes.[2] There are two identified isoenzymes, SK1 and SK2.[3][4][5] These two enzymes have different tissue distribution. SK1 is highly expressed in spleen, lung and leukocytes,[3] while SK2 is highly expressed in liver and kidney.[3] SK2 is located mainly in the mitochondria, nucleus and the endoplasmic reticulum whereas SK1 is mainly located in cytoplasm and the cell membrane.[6][7][8]
Metabolism and degradation
S1P can be dephosphorylated to sphingosine by sphingosine phosphatases and can be irreversibly degraded by an enzyme, sphingosine phosphate lyase.
Function
S1P is a blood borne lipid mediator, in particular in association with lipoproteins such as high density lipoprotein (HDL).[9] It is less abundant in tissue fluids. This is referred to as the S1P gradient, which seems to have biological significance in immune cell trafficking.
Originally thought as an intracellular second messenger, it was discovered to be an extracellular ligand for G protein-coupled receptor S1PR1 in 1998. It is now known that sphingosine-1-phosphate receptors (S1P receptors) are members of the lysophospholipid receptor family. There are five described to date. Most of the biological effects of S1P are mediated by signaling through the cell surface receptors.
Although S1P is of importance in the entire human body, it is a major regulator of vascular and immune systems. In addition, it might be relevant in the skin. In the vascular system, S1P regulates angiogenesis, vascular stability, and permeability. In the immune system, it is now recognized as a major regulator of trafficking of T- and B-cells. S1P interaction with its receptor S1PR1 is needed for the egress of immune cells from the lymphoid organs (such as thymus and lymph nodes) into the lymphatic vessels. Inhibition of S1P receptors was shown to be critical for immunomodulation. S1P has also been shown to directly suppress TLR mediated immune response from T cells.[10]
A research team, led by a scientist at Weill Cornell Medical College, has discovered that red blood cells perform a second vital function: angiogenesis. Given its role in creating new blood vessels, scientists recognize S1P as vital to human health — and a player in some diseases, such as cancer. And although S1P is known to be blood borne, no one realized until this study that S1P is supplied by red blood cells to control blood vessel growth.
Clinical significance
The levels of S1P (in a range of 5–40 μmol/L) are 5 to 10 times up-regulated in ovarian cancer patients' ascites. S1P at this physiological concentration stimulates migration and invasion of epithelial ovarian cancer cells but inhibits migration of normal ovarian surface epithelial cells.[11] Most (more than 90%) ovarian cancers arise from the epithelium of the ovary. Therefore, extracellular S1P could have an important role in cancer progression by promoting migration of epithelial ovarian cancer cells.
Ozonization of human blood is associated with increased concentrations of S1P in the plasma.[12]
In addition, S1P modulates the proliferation of skin cells. This in particular applies to keratinocytes[13] while fibroblasts are not addressed in this way, apart from cell growth and differentiation. While S1P suppresses epidermal proliferation as the glucocorticoids do, it differs from them in so far, as proliferation of dermal fibroblasts is not reduced. In fact, S1P even activates fibroblast-derived extracellular matrix protein production.
As a drug
Administration of S1P has been shown to protect oocytes from chemotherapeutic agents in vitro,[14][15][16] as well as in vivo from chemotherapeutic and radiation therapies.[14][17][18][19] which otherwise induce apoptosis of the cells. S1P has protected ovarian tissue xenografts in SCID mouse models from radiation induced atresia.[19] In animal models these protected oocytes have been used to produce healthy live young.[17][20] Radiotherapies and chemotherapies can cause apoptosis of ovarian follicles, causing premature ovarian failure,[21] and so S1P is of great interest in fertility preservation.[22] However, its mechanism of inhibiting the sphingomyelin apoptotic pathway may also interfere with the apoptosis action of chemotherapy drugs.[23]
Due to the hyperproliferative action against epidermal cells, S1P has been considered as an active pharmaceutical ingredient for hyperproliferative skin diseases, in particular, psoriasis vulgaris and acne vulgaris.
Although S1P is active at very low concentrations, bioavailability of the compound in human skin is a concern. Therefore, a topical formulation based on specific drug carriers has been considered inevitable.
As a drug target
Lpath Inc has produced and optimized a monoclonal anti-S1P antibody (Sphingomab). Sphingomab can absorb S1P from the extracellular fluid, thereby lowering the effective concentration of S1P.
Sonepcizumab is an experimental anti-S1P monoclonal antibody that has had a phase II clinical trial for renal cell carcinoma.[24] Sonepcizumab (LT1009) as ASONEP (for intravenous injection) has been studied for solid tumours.[25] As iSONEP, a formulation for intravitreal injection, it has been studied for age-related macular degeneration.[26]
S1P receptor(s) as a drug target
There are 5 types of Sphingosine-1-phosphate receptor.
S1P receptor modulators
The drug fingolimod (FTY720), which agonizes the S1P receptor,[27] prevents autoimmune lymphocytes from moving from the lymphoid organs into the central nervous system. It has been shown in phase III clinical trials to reduce relapses and improve other outcomes in multiple sclerosis.[28][29] S1P, as well as FTY720, has been shown to have anti-inflammatory properties at low concentrations and prevent monocyte:endothelial interactions in aorta, possibly through the S1P1 receptor.[30][31] The S1P receptor agonist etrasimod has been shown to induce remission in patient with ulcerative colitis.[32]
ONO-4641 (a drug of Ono Pharmaceutical Co., Ltd.) is a sphingosine-1-phosphate (S1P) receptor agonist which keeps lymphocytes in lymph nodes and thereby inhibits the infiltration of lymphocytes into lesions. The compound is therefore expected to be a drug for the treatment of auto-immune diseases such as multiple sclerosis, which is regarded as an intractable disease.
Ozanimod is an agonist of the S1P1 and S1P5 receptors.[33] and has been studied for various forms of multiple sclerosis.[34]
See also
Notes
- Mendelson, Karen; Evans, Todd; Hla, Timothy (1 January 2014). "Sphingosine 1-phosphate signalling". Development. 141 (1): 5–9. doi:10.1242/dev.094805. ISSN 0950-1991. PMC 3865745. PMID 24346695.
- Spiegel, Sarah; Milstien, Sheldon (26 January 2007). "Functions of the multifaceted family of sphingosine kinases and some close relatives". The Journal of Biological Chemistry. 282 (4): 2125–2129. doi:10.1074/jbc.R600028200. ISSN 0021-9258. PMID 17135245.
- Liu, H.; Sugiura, M.; Nava, V. E.; Edsall, L. C.; Kono, K.; Poulton, S.; Milstien, S.; Kohama, T.; Spiegel, S. (30 June 2000). "Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform". The Journal of Biological Chemistry. 275 (26): 19513–19520. doi:10.1074/jbc.M002759200. ISSN 0021-9258. PMID 10751414.
- Maceyka, Michael; Sankala, Heidi; Hait, Nitai C.; Le Stunff, Hervé; Liu, Hong; Toman, Rachelle; Collier, Claiborne; Zhang, Min; Satin, Leslie S.; Merrill, Alfred H.; Milstien, Sheldon (4 November 2005). "SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism". The Journal of Biological Chemistry. 280 (44): 37118–37129. doi:10.1074/jbc.M502207200. ISSN 0021-9258. PMID 16118219.
- Liu, Hong; Chakravarty, Debyani; Maceyka, Michael; Milstien, Sheldon; Spiegel, Sarah (2002). "Sphingosine kinases: a novel family of lipid kinases". Progress in Nucleic Acid Research and Molecular Biology. 71: 493–511. doi:10.1016/s0079-6603(02)71049-0. ISBN 9780125400718. ISSN 0079-6603. PMID 12102559.
- Strub, Graham M.; Paillard, Melanie; Liang, Jie; Gomez, Ludovic; Allegood, Jeremy C.; Hait, Nitai C.; Maceyka, Michael; Price, Megan M.; Chen, Qun; Simpson, David C.; Kordula, Tomasz (February 2011). "Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration". FASEB Journal. 25 (2): 600–612. doi:10.1096/fj.10-167502. ISSN 1530-6860. PMC 3023391. PMID 20959514.
- Adams, David R.; Pyne, Susan; Pyne, Nigel J. (May 2016). "Sphingosine Kinases: Emerging Structure-Function Insights" (PDF). Trends in Biochemical Sciences. 41 (5): 395–409. doi:10.1016/j.tibs.2016.02.007. ISSN 0968-0004. PMID 27021309.
- Fukuda, Yu; Kihara, Akio; Igarashi, Yasuyuki (12 September 2003). "Distribution of sphingosine kinase activity in mouse tissues: contribution of SPHK1". Biochemical and Biophysical Research Communications. 309 (1): 155–160. doi:10.1016/s0006-291x(03)01551-1. ISSN 0006-291X. PMID 12943676.
- Sattler K, Levkau B (May 2009). "Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection". Cardiovascular Research. 82 (2): 201–11. doi:10.1093/cvr/cvp070. PMID 19233866.
- Sharma, N; et al. (2013). "Sphingosine-1-phosphate suppresses TLR-induced CXCL8 secretion from human T cells". J Leukoc Biol. 93 (4): 521–528. doi:10.1189/jlb.0712328. PMID 23345392.
- Wang, D; et al. (2008). "S1P differentially regulates migration of human ovarian cancer and human ovarian surface epithelial cells". Mol Cancer Ther. 7 (7): 1993–2002. doi:10.1158/1535-7163.MCT-08-0088. PMC 2649755. PMID 18645009.
- Boczkowska-Radziwon, B; Chabowska, AM; Blachnio-Zabielska, A; Lukaszuk, B; Lipska, A; Chabowski, A; Radziwon, P (April 2015). "Ozonation of human blood increases sphingosine-1-phosphate in plasma". Journal of Physiology and Pharmacology. 66 (2): 267–72. PMID 25903957.
- Manggau M, Kim DS, Ruwisch L, et al. (November 2001). "1Alpha,25-dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate". The Journal of Investigative Dermatology. 117 (5): 1241–9. doi:10.1046/j.0022-202x.2001.01496.x. PMID 11710939.
- Morita Y, Perez GI, Paris F, et al. (October 2000). "Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy". Nature Medicine. 6 (10): 1109–14. doi:10.1038/80442. PMID 11017141. S2CID 33216920.
- Jurisicova A, Lee HJ, D'Estaing SG, Tilly J, Perez GI (September 2006). "Molecular requirements for doxorubicin-mediated death in murine oocytes". Cell Death and Differentiation. 13 (9): 1466–74. doi:10.1038/sj.cdd.4401819. PMID 16439991.
- Perez, Gloria I.; Knudson, C. Michael; Leykin, Lucy; Korsmeyer, Stanley J.; Tilly, Jonathan L. (1 November 1997). "Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction". Nature Medicine. 3 (11): 1228–1232. doi:10.1038/nm1197-1228. PMID 9359697. S2CID 32735595.
- Paris F, Perez GI, Fuks Z, et al. (September 2002). "Sphingosine 1-phosphate preserves fertility in irradiated female mice without propagating genomic damage in offspring". Nature Medicine. 8 (9): 901–2. doi:10.1038/nm0902-901. PMID 12205432. S2CID 2647395.
- Kaya H, Desdicioglu R, Sezik M, et al. (March 2008). "Does sphingosine-1-phosphate have a protective effect on cyclophosphamide- and irradiation-induced ovarian damage in the rat model?". Fertility and Sterility. 89 (3): 732–5. doi:10.1016/j.fertnstert.2007.03.065. PMID 17517398.
- Zelinski MB, Murphy MK, Lawson MS, et al. (March 2011). "In vivo delivery of FTY720 prevents radiation-induced ovarian failure and infertility in adult female nonhuman primates". Fertility and Sterility. 95 (4): 1440–5.e1–7. doi:10.1016/j.fertnstert.2011.01.012. PMC 3063448. PMID 21316047.
- Hancke K, Strauch O, Kissel C, Göbel H, Schäfer W, Denschlag D (January 2007). "Sphingosine 1-phosphate protects ovaries from chemotherapy-induced damage in vivo". Fertility and Sterility. 87 (1): 172–7. doi:10.1016/j.fertnstert.2006.06.020. PMID 17081530.
- Byrne J, Fears TR, Gail MH, et al. (March 1992). "Early menopause in long-term survivors of cancer during adolescence". American Journal of Obstetrics and Gynecology. 166 (3): 788–93. doi:10.1016/0002-9378(92)91335-8. PMID 1550144.
- Blumenfeld, Zeev (1 September 2012). "Preservation of ovarian function and fertility despite gonadotoxic chemotherapy". Expert Review of Endocrinology & Metabolism. 7 (5): 567–576. doi:10.1586/eem.12.40. PMID 30780892. S2CID 59105653.
- Roness, H.; Kalich-Philosoph, L.; Meirow, D. (2014). "Prevention of chemotherapy-induced ovarian damage: possible roles for hormonal and non-hormonal attenuating agents". Human Reproduction Update. 20 (5): 759–774. doi:10.1093/humupd/dmu019. ISSN 1355-4786. PMID 24833728.
- "Sonepcizumab Failed PFS Endpoint, but Had Promising OS, in Metastatic RCC".
- Safety Study of ASONEP (Sonepcizumab/LT1009) to Treat Advanced Solid Tumors (ASONEP)
- Safety Study of iSONEP (Sonepcizumab/LT1009) to Treat Neovascular Age-related Macular Degeneration
- Baumrucker, T; et al. (2007). "FTY720, an immunomodulatory sphingolipid mimetic: translation of a novel mechanism into clinical benefit in multiple sclerosis". Expert Opin Investig Drugs. 16 (3): 283–289. doi:10.1517/13543784.16.3.283. PMID 17302523. S2CID 23289454.
- Kappos L, Radue EW, O'Connor P, et al. (February 2010). "A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis". The New England Journal of Medicine. 362 (5): 387–401. doi:10.1056/NEJMoa0909494. hdl:11858/00-001M-0000-0012-1FF5-A. PMID 20089952.
- Cohen JA, Barkhof F, Comi G, et al. (February 2010). "Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis". The New England Journal of Medicine. 362 (5): 402–15. doi:10.1056/NEJMoa0907839. hdl:2078.1/124998. PMID 20089954.
- Bolick DT, Srinivasan S, Kim KW, et al. (May 2005). "Sphingosine-1-Phosphate Prevents Tumor Necrosis Factor-α–Mediated Monocyte Adhesion to Aortic Endothelium in Mice". Arteriosclerosis, Thrombosis, and Vascular Biology. 25 (5): 976–81. doi:10.1161/01.ATV.0000162171.30089.f6. PMID 15761190.
- Whetzel AM, Bolick DT, Srinivasan S, et al. (September 2006). "Sphingosine-1 phosphate prevents monocyte/endothelial interactions in type 1 diabetic NOD mice through activation of the S1P1 receptor". Circulation Research. 99 (7): 731–9. doi:10.1161/01.RES.0000244088.33375.52. PMID 16960101.
- Sandborn, William J; Vermeire, Séverine; Peyrin-Biroulet, Laurent; Dubinsky, Marla C; Panes, Julian; Yarur, Andres; Ritter, Timothy; Baert, Filip; Schreiber, Stefan; Sloan, Sheldon; Cataldi, Fabio; Shan, Kevin; Rabbat, Christopher J; Chiorean, Michael; Wolf, Douglas C; Sands, Bruce E; D'Haens, Geert; Danese, Silvio; Goetsch, Martina; Feagan, Brian G (April 2023). "Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): two randomised, double-blind, placebo-controlled, phase 3 studies". The Lancet. 401 (10383): 1159–1171. doi:10.1016/S0140-6736(23)00061-2.
- Scott, F L; Clemons, B; Brooks, J; Brahmachary, E; Powell, R; Dedman, H; Desale, H G; Timony, G A; Martinborough, E (1 June 2016). "Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity". British Journal of Pharmacology. 173 (11): 1778–1792. doi:10.1111/bph.13476. ISSN 1476-5381. PMC 4867749. PMID 26990079.
- New Frontiers in S1P Modulators. March 2017
References
- Lee MJ, Van Brocklyn JR, Thangada S, et al. (March 1998). "Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1". Science. 279 (5356): 1552–5. Bibcode:1998Sci...279.1552L. doi:10.1126/science.279.5356.1552. PMID 9488656.
- Bollag WB (April 2003). "Paradoxical effects of sphingosine-1-phosphate". The Journal of Investigative Dermatology. 120 (4): xiii–xiv. doi:10.1046/j.1523-1747.2003.12116.x. PMID 12648243.
- Vogler R, Sauer B, Kim DS, Schäfer-Korting M, Kleuser B (April 2003). "Sphingosine-1-phosphate and its potentially paradoxical effects on critical parameters of cutaneous wound healing". The Journal of Investigative Dermatology. 120 (4): 693–700. doi:10.1046/j.1523-1747.2003.12096.x. PMID 12648236.
- MS Drug ONO-4641 Slows Brain Legions By 92%
Further reading
- Xie, B.; Shen, J.; Dong, A.; Rashid, A.; Stoller, G.; Campochiaro, P. A. (2009). "Blockade of Sphingosine-1-phosphate Reduces Macrophage Influx and Retinal and Choroidal Neovascularization". Journal of Cellular Physiology. 218 (1): 192–198. doi:10.1002/jcp.21588. PMC 2905312. PMID 18781584.