Spiculisporic acid
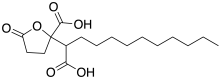
Spiculisporic acid is a bioactive γ-butenolide. It was originally isolated from Penicillium spiculisporum.[1] Structural variants have been isolated from a marine Aspergillus.[2]
 | |
| Names | |
|---|---|
| IUPAC name
2-(1-Carboxyundecyl)-5-oxooxolane-2-carboxylic acid | |
| Other names
4,5-Dicarboxy-gamma-pentadecanolactone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C17H28O6 | |
| Molar mass | 328.405 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Notes
- Clutterbuck; Rastrick; Rintoul (1931). "Studies in the Biochemistry of Micro-organisms. Part XVI— on the production from Glucose by Penicillium Spiculisporum Lehman of a new polybasic fatty acid, C 17 H 28 O 6 (The lactone of γ-hydroxy-βδ-dicarboxypentadecoic acid)". Phil. Trans. R. Soc. Lond. B. 220 (468–473): 301. doi:10.1098/rstb.1931.0027.
- Wang, R; Liu, TM; Shen, MH; Yang, MQ; Feng, QY; Tang, XM; Li, XM (2012). "Spiculisporic acids B–D, three new γ-butenolide derivatives from a sea urchin-derived fungus Aspergillus sp. HDf2". Molecules (Basel, Switzerland). 17 (11): 13175–82. doi:10.3390/molecules171113175. PMC 6268229. PMID 23128094.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.