Stabilizing selection
Stabilizing selection (not to be confused with negative or purifying selection[1][2]) is a type of natural selection in which the population mean stabilizes on a particular non-extreme trait value. This is thought to be the most common mechanism of action for natural selection because most traits do not appear to change drastically over time.[3] Stabilizing selection commonly uses negative selection (a.k.a. purifying selection) to select against extreme values of the character. Stabilizing selection is the opposite of disruptive selection. Instead of favoring individuals with extreme phenotypes, it favors the intermediate variants. Stabilizing selection tends to remove the more severe phenotypes, resulting in the reproductive success of the norm or average phenotypes.[4] This means that most common phenotype in the population is selected for and continues to dominate in future generations.
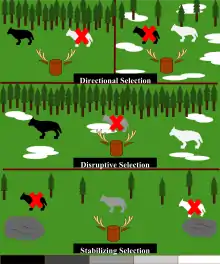
.svg.png.webp)
History
The Russian evolutionary biologist Ivan Schmalhausen founded the theory of stabilizing selection, publishing a paper in Russian titled "Stabilizing selection and its place among factors of evolution" in 1941 and a monograph "Factors of Evolution: The Theory of Stabilizing Selection" in 1945.[5][6]
Influence on population structure
Stabilizing selection causes the narrowing of the phenotypes seen in a population. This is because the extreme phenotypes are selected against, causing reduced survival in organisms with those traits. This results in a population consisting of fewer phenotypes, with most traits representing the mean value of the population. This narrowing of phenotypes causes a reduction in genetic diversity in a population.[7] Maintaining genetic variation is essential for the survival of a population because it is what allows them to evolve over time. In order for a population to adapt to changing environmental conditions they must have enough genetic diversity to select for new traits as they become favorable.[8]
Analyzing stabilizing selection
There are four primary types of data used to quantify stabilizing selection in a population. The first type of data is an estimation of fitness of different phenotypes within a single generation. Quantifying fitness in a single generation creates predictions for the expected fate of selection. The second type of data is changes in allelic frequencies or phenotypes across different generations. This allows quantification of change in prevalence of a certain phenotype, indicating the type of selection. The third type of data is differences in allelic frequencies across space. This compares selection occurring in different populations and environmental conditions. The fourth type of data is DNA sequences from the genes contributing to observes phenotypic differences. The combination of these four types of data allow population studies that can identify the type of selection occurring and quantify the extent of selection.[9]
However, a meta-analysis of studies that measured selection in the wild failed to find an overall trend for stabilizing selection.[10] The reason can be that methods for detecting stabilizing selection are complex. They can involve studying the changes that causes natural selection in the mean and variance of the trait, or measuring fitness for a range of different phenotypes under natural conditions and examining the relationship between these fitness measurements and the trait value, but analysis and interpretation of the results is not straightforward.[11]
Examples
The most common form of stabilizing selection is based on phenotypes of a population. In phenotype based stabilizing selection, the mean value of a phenotype is selected for, resulting a decrease in the phenotypic variation found in a population.[12]
Humans
Stabilizing selection is the most common form of nonlinear selection (non-directional) in humans.[13] There are few examples of genes with direct evidence of stabilizing selection in humans. However, most quantitative traits (height, birthweight, schizophrenia) are thought to be under stabilizing selection, due to their polygenicity and the distribution of the phenotypes throughout human populations.[14]
- Birth Weight − A classic example of this is human birth weight. Babies of low weight lose heat more quickly and get ill from infectious diseases more easily, whereas babies of large body weight are more difficult to deliver through the pelvis. Infants of a more medium weight survive much more often. For the larger or smaller babies, the baby mortality rate is much higher.[15] The bell curve of the human population peaks at a birth weight that the newly born babies exhibit the minimum death rate.
Plants
- Height − Another example of a trait, that might be acted on by stabilizing selection, is plant height. A plant that is too short may not be able to compete with other plants for sunlight. However, extremely tall plants may be more susceptible to wind damage. Combined, these two selection pressures select to maintain plants of medium height. The number of plants of medium height will increase while the numbers of short and tall plants will decrease.[16]
- Cacti Spine Number − Desert populations of spiny cacti experience predation by peccaries, which consume the fleshy part of the cactus. This can be prevented by increasing the number of spines on the cactus. However, there is also a selection pressure in the opposite direction because there is a parasitic insect that will lay its eggs in spines if they are densely populated. This means that in order to manage both of these selection pressures the cacti experiences stabilizing selection to balance the appropriate number of spines to survive these different threats.[17]
Insects
- Butterfly's Winged Eyespots − The African butterfly Bicyclus anynana exhibits stabilizing selection with its wing eyespots.[18] It has been suggested that the circular eyespots positioned on the wings are favoured functionally compared to other shapes and sizes.[19]
.jpg.webp) Bicyclus anynana with wing eyespot, which experiences stabilizing selection to avoid predation.
Bicyclus anynana with wing eyespot, which experiences stabilizing selection to avoid predation. - Gall Size − The Eurosta solidaginis fly lays its eggs on the tip of plants, which then encase the larvae in a protective gall. The size of this gall is under stabilizing selection, as determined by predation. These larvae are under threat from parasitic wasps, which lay a single egg in galls containing the flies. The single wasp offspring then consumes the fly larvae to survive. Therefore, a larger gall is favored to allow more places for larvae to hide from the wasp. However, larger galls attract a different type of predation from birds, as they can penetrate large galls with their beak. Therefore, the optimal gall is moderately sized in order to avoid predation from both birds and wasps.[20]
Birds
- Clutch Size − The number of eggs laid by a female bird (clutch size) is typically under stabilizing selection. This is because the female must lay as many eggs as possible to maximize the number of offspring. However, they can only lay as many eggs as they can support with their own resources. Laying too many eggs could expend all of the energy of the mother bird causing her to die and the death of the chicks. Additionally, once the eggs hatch the mother must be able to obtain enough resources to keep all of the chicks alive. Therefore, the mother typically lays a moderate amount of eggs in order to increase offspring survival and maximize the number of offspring.[21]
Mammals
- The Siberian husky experiences stabilizing selection in terms of their leg muscles. These dogs have to have enough muscle in order to pull sleds and move quickly. However, they also must be light enough to stay on top of the snow. This means that the leg muscles of the husky are most fit when they are moderately sized, to balance their strength and their weight.[22]
 The Siberian husky experiences stabilizing selection in terms of their leg muscles, allowing them to be strong but light.
The Siberian husky experiences stabilizing selection in terms of their leg muscles, allowing them to be strong but light.
See also
References
- Lemey P, Salemi M, Vandamme A (2009). The Phylogenetic Handbook. Cambridge University Press. ISBN 978-0-521-73071-6.
- Loewe L. "Negative Selection". Nature Education. 1 (1): 59.
- Charlesworth B, Lande R, Slatkin M (May 1982). "A neo-Darwinian commentary on macroevolution". Evolution; International Journal of Organic Evolution. 36 (3): 474–498. doi:10.1111/j.1558-5646.1982.tb05068.x. JSTOR 2408095. PMID 28568049. S2CID 27361293.
- Campbell NA, Reece JB (2002). Biology. Benjamin Cummings. pp. 450–451. ISBN 9780805366242.
- Levit GS, Hossfeld U, Olsson L (March 2006). "From the "Modern Synthesis" to cybernetics: Ivan Ivanovich Schmalhausen (1884-1963) and his research program for a synthesis of evolutionary and developmental biology". Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. Wiley-Liss. 306 (2): 89–106. doi:10.1002/jez.b.21087. PMID 16419076. S2CID 23594114.
- Adams MB (June 1988). "A Missing Link in the Evolutionary Synthesis. I. I. Schmalhausen. Factors of Evolution: The Theory of Stabilizing Selection". Isis. 79 (297): 281–284. doi:10.1086/354706. PMID 3049441. S2CID 146660877.
- Hunt J, Blows MW, Zajitschek F, Jennions MD, Brooks R (October 2007). "Reconciling strong stabilizing selection with the maintenance of genetic variation in a natural population of black field crickets (Teleogryllus commodus)". Genetics. 177 (2): 875–80. doi:10.1534/genetics.107.077057. PMC 2034650. PMID 17660544.
- "Low genetic variation". evolution.berkeley.edu. Retrieved 2018-05-13.
- Linnen CR, Hoekstra HE (2009). "Measuring natural selection on genotypes and phenotypes in the wild". Cold Spring Harbor Symposia on Quantitative Biology. 74: 155–68. doi:10.1101/sqb.2009.74.045. PMC 3918505. PMID 20413707.
- Kingsolver JG, Hoekstra HE, Hoekstra J, Berrigan D, Vignieri SN, Hill CE, Hoang A, Gilbert P, Beerli P (2001). "The Strength of Super Genetic Selection in Natural Populations" (PDF). The American Naturalist. 157 (3): 245–61. doi:10.1086/319193. PMID 18707288. S2CID 11408433.
- Lande R, Arnold SJ (November 1983). "The Measurement of Selection on Correlated Characters". Evolution; International Journal of Organic Evolution. 37 (6): 1210–1226. doi:10.1111/j.1558-5646.1983.tb00236.x. PMID 28556011. S2CID 36544045.
- Kingsolver JG, Diamond SE (March 2011). "Phenotypic selection in natural populations: what limits directional selection?". The American Naturalist. 177 (3): 346–57. doi:10.1086/658341. PMID 21460543. S2CID 26806172.
- Sanjak JS, Sidorenko J, Robinson MR, Thornton KR, Visscher PM (January 2018). "Evidence of directional and stabilizing selection in contemporary humans". Proceedings of the National Academy of Sciences of the United States of America. 115 (1): 151–156. Bibcode:2018PNAS..115..151S. doi:10.1073/pnas.1707227114. PMC 5776788. PMID 29255044.
- Simons YB, Bullaughey K, Hudson RR, Sella G (March 16, 2018). "A population genetic interpretation of GWAS findings for human quantitative traits". PLOS Biology. 16 (3): e2002985. arXiv:1704.06707. doi:10.1371/journal.pbio.2002985. PMC 5871013. PMID 29547617.
- Carr SM (2004). "Stabilizing Selection on birthweight in humans".
- "Natural Selection". SparkNotes.
- "Stabilizing Selection". www.brooklyn.cuny.edu. Retrieved 2018-05-13.
- Brakefield PM, Beldade P, Zwaan BJ (May 2009). "The African butterfly Bicyclus anynana: a model for evolutionary genetics and evolutionary developmental biology". Cold Spring Harbor Protocols. 2009 (5): pdb.emo122. doi:10.1101/pdb.emo122. PMID 20147150.
- Brakefield PM (March 1998). "The evolution–development interface and advances with the eyespot patterns of Bicyclus butterflies". Heredity. 80 (3): 265–272. doi:10.1046/j.1365-2540.1998.00366.x.
- László Z, Sólyom K, Prázsmári H, Barta Z, Tóthmérész B (2014-06-11). "Predation on rose galls: parasitoids and predators determine gall size through directional selection". PLOS ONE. 9 (6): e99806. Bibcode:2014PLoSO...999806L. doi:10.1371/journal.pone.0099806. PMC 4053394. PMID 24918448.
- "Variation in Clutch Sizes". web.stanford.edu. Retrieved 2018-05-13.
- "A Simple Definition and Prominent Examples of Stabilizing Selection". BiologyWise. Retrieved 2018-05-16.