Stigmatellin
Stigmatellin is a potent inhibitor of the quinol oxidation (Qo) site of the cytochrome bc1 complex in mitochondria[1] and the cytochrome b6f complex of thylakoid membranes. At higher concentrations, stigmatellin also inhibits Complex I, as a "Class B" inhibitor of that enzyme.[2]
 | |
| Names | |
|---|---|
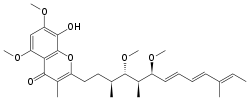
| Preferred IUPAC name
2-[(3S,4S,5S,6S,7E,9E,11E)-4,6-Dimethoxy-3,5,11-trimethyltrideca-7,9,11-trien-1-yl]-8-hydroxy-5,7-dimethoxy-3-methyl-4H-1-benzopyran-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.149.842 |
| MeSH | Stigmatellin |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C30H42O7 | |
| Molar mass | 514.65 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Stigmatellin is isolated from the myxobacterium Stigmatella aurantica, and contains a 5,7-dimethoxy-8-hydroxychromone aromatic headgroup with a hydrophobic alkenyl chain in position 2. Crystal structures for stigmatellin-inhibited bc1 complex from bovine, avian, yeast (Saccharomyces cerevisiae) and bacterial (Rhodobacter capsulatus, Cereibacter sphaeroides, and Paracoccus denitrificans) sources are available. Stigmatellin binds at the cytochrome b Qo site in the '(heme) bl distal' position, and associates with the Rieske iron-sulfur protein via a hydrogen bond to histidine residue 181 (His-181), a ligand to the [2Fe2S] iron-sulfur cluster of this subunit. This association raises the midpoint potential of the iron-sulfur cluster from 290 to 540 mV and restricts movement of the cytoplasmic domain of the Rieske protein.
References
- von Jagow G, Ohnishi T (June 1985). "The chromone inhibitor stigmatellin--binding to the ubiquinol oxidation center at the C-side of the mitochondrial membrane". FEBS Letters. 185 (2): 311–5. doi:10.1016/0014-5793(85)80929-7. PMID 2987042.
- Fato R, Bergamini C, Bortolus M, Maniero AL, Leoni S, Ohnishi T, Lenaz G (May 2009). "Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1787 (5): 384–92. doi:10.1016/j.bbabio.2008.11.003. PMC 2724837. PMID 19059197.
Further reading
- von Jagow G, Link TA (1986). Use of specific inhibitors on the mitochondrial bc1 complex. Methods in Enzymology. Vol. 126. pp. 253–71. doi:10.1016/s0076-6879(86)26026-7. PMID 2856132.