Stratospheric aerosol injection
Stratospheric aerosol injection is a proposed method of solar geoengineering (or solar radiation modification) to reduce global warming. This would introduce aerosols into the stratosphere to create a cooling effect via global dimming and increased albedo, which occurs naturally from volcanic winter.[1] It appears that stratospheric aerosol injection, at a moderate intensity, could counter most changes to temperature and precipitation, take effect rapidly, have low direct implementation costs, and be reversible in its direct climatic effects.[2] The Intergovernmental Panel on Climate Change concludes that it "is the most-researched [solar geoengineering] method, with high agreement that it could limit warming to below 1.5 °C (2.7 °F)."[3] However, like other solar geoengineering approaches, stratospheric aerosol injection would do so imperfectly and other effects are possible,[4] particularly if used in a suboptimal manner.[5]
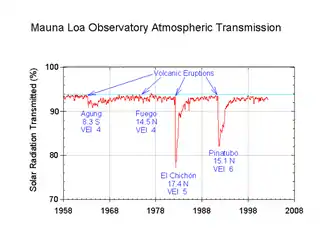
Various forms of sulfur have been shown to cool the planet after large volcanic eruptions.[6] However, as of 2021, there has been little research and existing natural aerosols in the stratosphere are not well understood.[7] so there is no leading candidate material. Alumina, calcite and salt are also under consideration.[8][9] The leading proposed method of delivery is custom aircraft.[10]
Scientific basis
Natural and anthropogenic sulfates
There is a wide range of particulate matter suspended in the atmosphere at various height and in various sizes. By far the best-studied are the various sulfur compounds collectively referred to sulfate aerosols. This group includes inorganic sulfates (SO42-),HSO4- and H2SO4-: organic sulfur compounds are sometimes included as well, but are of lower importance.[11] Sulfate aerosols can be anthropogenic (through the combustion of fossil fuels with a high sulfur content, primarily coal and certain less-refined fuels, like aviation and bunker fuel),[12][13] biogenic from hydrosphere and biosphere, geological via volcanoes or weather-driven from wildfires and other natural combustion events.[14][15][13]
Inorganic aerosols are mainly produced when sulfur dioxide reacts with water vapor to form gaseous sulfuric acid and various salts (often through an oxidation reaction in the clouds), which are then thought to experience hygroscopic growth and coagulation and then shrink through evaporation.[16][14] as microscopic liquid droplets or fine (diameter of about 0.1 to 1.0 micrometre) sulfate solid particles in a colloidal suspension,[17][15] with smaller particles at times coagulating into larger ones.[18]The other major source are chemical reactions with dimethyl sulfide (DMS), predominantly sourced from marine plankton, with a smaller contribution from swamps and other such wetlands.[17] And sometimes, aerosols are produced from photochemical decomposition of COS (carbonyl sulfide), or when solid sulfates in the sea salt spray can react with gypsum dust particles).

Major volcanic eruptions have an overwhelming effect on sulfate aerosol concentrations in the years when they occur: eruptions ranking 4 or greater on the Volcanic Explosivity Index inject SO2 and water vapor directly into the stratosphere, where they react to create sulfate aerosol plumes.[19] Volcanic emissions vary significantly in composition, and have complex chemistry due to the presence of ash particulates and a wide variety of other elements in the plume. Only stratovolcanoes containing primarily felsic magmas are responsible for these fluxes, as mafic magma erupted in shield volcanoes doesn't result in plumes which reach the stratosphere.[20] However, before the Industrial Revolution, dimethyl sulfide pathway was the largest contributor to sulfate aerosol concentrations in a more average year with no major volcanic activity. According to the IPCC First Assessment Report, published in 1990, volcanic emissions usually amounted to around 10 million tons in 1980s, while dimethyl sulfide amounted to 40 million tons. Yet, by that point, the global human-caused emissions of sulfur into the atmosphere became "at least as large" as all natural emissions of sulfur-containing compounds combined: they were at less than 3 million tons per year in 1860, and then they increased to 15 million tons in 1900, 40 million tons in 1940 and about 80 millions in 1980. The same report noted that "in the industrialized regions of Europe and North America, anthropogenic emissions dominate over natural emissions by about a factor of ten or even more".[21] In the eastern United States, sulfate particles were estimated to account for 25% or more of all air pollution.[22] Meanwhile, the Southern Hemisphere had much lower concentrations due to being much less densely populated, with an estimated 90% of the human population in the north. In the early 1990s, anthropogenic sulfur dominated in the Northern Hemisphere, where only 16% of annual sulfur emissions were natural, yet amounted for less than half of the emissions in the Southern Hemisphere.[23]

Such an increase in sulfate aerosol emissions had a variety of effects. At the time, the most visible one was acid rain, caused by precipitation from clouds carrying high concentrations of sulfate aerosols in the troposphere.[24]
At its peak, acid rain has eliminated brook trout and some other fish species and insect life from lakes and streams in geographically sensitive areas, such as Adirondack Mountains in the United States.[25] Acid rain worsens soil function as some of its microbiota is lost and heavy metals like aluminium are mobilized (spread more easily) while essential nutrients and minerals such as magnesium can leach away because of the same. Ultimately, plants unable to tolerate lowered pH are killed, with montane forests being some of the worst-affected ecosystems due to their regular exposure to sulfate-carrying fog at high altitudes.[26][27][28][29][30] While acid rain was too dilute to affect human health directly, breathing smog or even any air with elevated sulfate concentrations is known to contribute to heart and lung conditions, including asthma and bronchitis.[22] Further, this form of pollution is linked to preterm birth and low birth weight, with a study of 74,671 pregnant women in Beijing finding that every additional 100 µg/m3 of SO2 in the air reduced infants' weight by 7.3 g, making it and other forms of air pollution the largest attributable risk factor for low birth weight ever observed.[31]Pollution controls and the discovery of radiative effects
.jpg.webp)
The discovery of these negative effects spurred the rush to reduce atmospheric sulfate pollution, typically through flue-gas desulfurization installations at power plants, such as wet scrubbers or fluidized bed combustion.[32][33] In the United States, this began with the passage of the Clean Air Act in 1970, which was strengthened in 1977 and 1990.[34] According to the EPA, from 1970 to 2005, total emissions of the six principal air pollutants, including sulfates, dropped by 53% in the US. By 2010, it valued the healthcare savings from these reductions at $50 billion annually.[35][36] In Europe, it was estimated in 2021 that the 18 coal-fired power plants in the western Balkans which lack controls on sulfur dioxide pollution have emitted two-and-half times more of it than all 221 coal plants in the European Union which are fitted with these technologies.[37] Globally, the uptake of treaties such as the 1985 Helsinki Protocol on the Reduction of Sulfur Emissions and its successors had gradually spread from the developed to the developing countries.[38] While China and India have seen decades in rapid growth of sulfur emissions while they declined in the U.S. and Europe, they have also peaked in the recent years. In 2005, China was the largest polluter, with its estimated 25,490,000 short tons (23.1 Mt) emissions increasing by 27% since 2000 alone and roughly matching the U.S. emissions in 1980.[39] That year was also the peak, and a consistent decline was recorded since then.[40] Similarly, India's sulfur dioxide emissions appear to have been largely flat in the 2010s, as more coal-fired power plants were fitted with pollution controls even as the newer ones were still coming online.[41]

Yet, around the time these treaties and technology improvements were taking place, evidence was coming in that sulfate aerosols were affecting both the visible light received by the Earth and its surface temperature. On one hand, the study of volcanic eruptions,[42] notably 1991 eruption of Mount Pinatubo in the Philippines,[43] [44] had shown that the mass formation of sulfate aerosols by these eruptions formed a subtle whitish haze in the sky,[45] reducing the amount of Sun's radiation reaching the Earth's surface and rapidly losing the heat they absorb back to space, as well increasing clouds' albedo (i.e. making them more reflective) by changing their consistency to a larger amount of smaller droplets,[12] which was the principal reason for a clear drop in global temperatures for several years in their wake.[46] On the other hand, multiple studies have shown that between 1950s and 1980s, the amount of sunlight reaching the surface declined by around 4–5% per decade,[47][48][49] even though the changes in solar radiation at the top of the atmosphere were never more than 0.1-0.3%.[50] Yet, this trend (commonly described as global dimming) began to reverse in the 1990s, consistent with the reductions in anthropogenic sulfate pollution,[51][52][53] while at the same time, climate change accelerated.[54][55] Areas like eastern United States went from seeing cooling in contrast to the global trend to becoming global warming hotspots as their enormous levels of air pollution were reduced,[56] even as sulfate particles still accounted for around 25% of all particulates.[36][57][58]

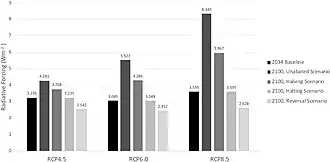
As the real world had shown the importance of sulfate aerosol concentrations to the global climate, research into the subject accelerated. Formation of the aerosols and their effects on the atmosphere can be studied in the lab, with methods like ion-chromatography and mass spectrometry[59] Samples of actual particles can be recovered from the stratosphere using balloons or aircraft, [60] and remote satellites were also used for observation.[61] This data is fed into the climate models,[62] as the necessity of accounting for aerosol cooling to truly understand the rate and evolution of warming had long been apparent, with the IPCC Second Assessment Report being the first to include an estimate of their impact on climate, and every major model able to simulate them by the time IPCC Fourth Assessment Report was published in 2007.[63] Many scientists also see the other side of this research, which is learning how to cause the same effect artificially.[64] While discussed around the 1990s, if not earlier,[65] stratospheric aerosol injection as a solar geoengineering method is best associated with Paul Crutzen's detailed 2006 proposal.[1] Deploying in the stratosphere ensures that the aerosols are at their most effective, and that the progress of clean air measures would not be reversed: more recent research estimated that even under the highest-emission scenario RCP 8.5, the addition of stratospheric sulfur required to avoid 4 °C (7.2 °F) relative to now (and 5 °C (9.0 °F) relative to the preindustrial) would be effectively offset by the future controls on tropospheric sulfate pollution, and the amount required would be even less for less drastic warming scenarios.[66] This spurred a detailed look at its costs and benefits,[67] but even with hundreds of studies into the subject completed by the early 2020s, some notable uncertainties remain.[68]
Methods
Materials

Various forms of sulfur were proposed as the injected substance, as this is in part how volcanic eruptions cool the planet.[6] Precursor gases such as sulfur dioxide and hydrogen sulfide have been considered. According to estimates, "one kilogram of well placed sulfur in the stratosphere would roughly offset the warming effect of several hundred thousand kilograms of carbon dioxide."[69] One study calculated the impact of injecting sulfate particles, or aerosols, every one to four years into the stratosphere in amounts equal to those lofted by the volcanic eruption of Mount Pinatubo in 1991,[70] but did not address the many technical and political challenges involved in potential solar geoengineering efforts.[71] Use of gaseous sulfuric acid appears to reduce the problem of aerosol growth.[10] Materials such as photophoretic particles, titanium dioxide, and diamond are also under consideration.[18][72][73]
Delivery
Various techniques have been proposed for delivering the aerosol or precursor gases.[1] The required altitude to enter the stratosphere is the height of the tropopause, which varies from 11 kilometres (6.8 mi/36,000 ft) at the poles to 17 kilometers (11 mi/58,000 ft) at the equator.

- Civilian aircraft including the Boeing 747–400 and Gulfstream G550/650, C-37A could be modified at relatively low cost to deliver sufficient amounts of required material according to one study,[74] but a later metastudy suggests a new aircraft would be needed but easy to develop.[75]
- Military aircraft such as the F15-C variant of the F-15 Eagle have the necessary flight ceiling, but limited payload. Military tanker aircraft such as the KC-135 Stratotanker and KC-10 Extender also have the necessary ceiling and have greater payload capacity.[76]
- Modified artillery might have the necessary capability,[77] but requires a polluting and expensive propellant charge to loft the payload. Railgun artillery could be a non-polluting alternative.
- High-altitude balloons can be used to lift precursor gases, in tanks, bladders or in the balloons' envelope.
Injection system
The latitude and distribution of injection locations has been discussed by various authors. Whilst a near-equatorial injection regime will allow particles to enter the rising leg of the Brewer-Dobson circulation, several studies have concluded that a broader, and higher-latitude, injection regime will reduce injection mass flow rates and/or yield climatic benefits.[78][79] Concentration of precursor injection in a single longitude appears to be beneficial, with condensation onto existing particles reduced, giving better control of the size distribution of aerosols resulting.[80] The long residence time of carbon dioxide in the atmosphere may require a millennium-timescale commitment to aerosol injection[81] if aggressive emissions abatement is not pursued simultaneously.
Advantages of the technique
The advantages of this approach in comparison to other possible means of solar geoengineering are:
- Mimics a natural process:[83] Stratospheric sulfur aerosols are created by existing natural processes (especially volcanoes), whose impacts have been studied via observations.[84] This contrasts with other, more speculative solar geoengineering techniques which do not have natural analogs (e.g., space sunshade).
- Technological feasibility: In contrast to other proposed solar geoengineering techniques, such as marine cloud brightening, much of the required technology is pre-existing: chemical manufacturing, artillery shells, high-altitude aircraft, weather balloons, etc.[6] Unsolved technical challenges include methods to deliver the material in controlled diameter with good scattering properties.
- Scalability: Some solar geoengineering techniques, such as cool roofs and ice protection, can only provide a limited intervention in the climate due to insufficient scale—one cannot reduce the temperature by more than a certain amount with each technique. Research has suggested that this technique may have a high radiative 'forcing potential'.[85], yet can be finely tuned according to how much cooling is needed.[82]
- Speed: A common argument is that stratospheric aerosol injection can take place quickly,[86] and would be able to buy time for carbon sequestration projects such as carbon dioxide air capture to be implemented and start acting over decades and centuries.[70]
Uncertainties
It is uncertain how effective any solar geoengineering technique would be, due to the difficulties modeling their impacts and the complex nature of the global climate system. Certain efficacy issues are specific to stratospheric aerosols.
- Lifespan of aerosols: Tropospheric sulfur aerosols are short-lived.[87] Delivery of particles into the lower stratosphere in the arctic will typically ensure that they remain aloft only for a few weeks or months, as air in this region is predominantly descending. To ensure endurance, higher-altitude delivery is needed, ensuring a typical endurance of several years by enabling injection into the rising leg of the Brewer-Dobson circulation above the tropical tropopause. Further, sizing of particles is crucial to their endurance.[88]
- Aerosol delivery: There are two proposals for how to create a stratospheric sulfate aerosol cloud, either through the release of a precursor gas (SO
2) or the direct release of sulfuric acid (H
2SO
4) and these face different challenges.[89] If SO
2 gas is released it will oxidize to form H
2SO
4 and then condense to form droplets far from the injection site.[90] Releasing SO
2 would not allow control over the size of the particles that are formed but would not require a sophisticated release mechanism. Simulations suggest that as the SO
2 release rate is increased there would be diminishing returns on the cooling effect, as larger particles would be formed which have a shorter lifetime and are less effective scatterers of light.[91] If H
2SO
4 is released directly then the aerosol particles would form very quickly and in principle the particle size could be controlled although the engineering requirements for this are uncertain. Assuming a technology for direct H
2SO
4 release could be conceived and developed, it would allow control over the particle size to possibly alleviate some of the inefficiencies associated with SO
2 release.[89] - Strength of cooling: The magnitude of the effect of forcing from aerosols by decreasing insolation received at the surface is not completely certain, as its scientific modelling involves complex calculations due to different confounding factors and parameters such as optical properties, spatial and temporal distribution of emission or injection, albedo, geography, loading, rate of transport of sulfate, global burden, atmospheric chemistry, mixing and reactions with other compounds and aerosols, particle size, relative humidity, and clouds. Along with others, aerosol size distribution and hygroscopicity have particularly high uncertainty due to being closely related to sulfate aerosol interactions with other aerosols which affects the amount of radiation reflected.[13][61] As of 2021, state-of-the-art CMIP6 models estimate that total cooling from the currently present aerosols is between 0.1 °C (0.18 °F) to 0.7 °C (1.3 °F);[92] the IPCC Sixth Assessment Report uses the best estimate of 0.5 °C (0.90 °F),[93] but there's still a lot of contradictory research on the impacts of aerosols of clouds which can alter this estimate of aerosol cooling, and consequently, our knowledge of how many millions of tons must be deployed annually to achieve the desired effect.[94][95][96][97][98][99][100]

- Hydrological cycle: Since the historical global dimming from tropospheric sulfate pollution is already well-known to have reduced rainfall in certain areas,[54][101] and is likely to have weakened Monsoon of South Asia[102] and contributed to or even outright caused the 1984 Ethiopian famine,[104][105][106] the impact on the hydrological cycle and patterns is one of the most-discussed uncertainties of the different stratospheric aerosol injection proposals.[107][108] It has been suggested that while changes in precipitation from stratospheric aerosol injection are likely to be more manageable than the changes expected under future warming, one of the main impacts it would have on mortality is by shifting the habitat of mosquitoes and thus substantially affecting the distribution and spread of vector-borne diseases. Considering the already-extensive present-day mosquito habitat, it is currently unclear whether those changes are likely to be positive or negative.[68]
Cost
Early studies suggest that stratospheric aerosol injection might have a relatively low direct cost. The annual cost of delivering 5 million tons of an albedo enhancing aerosol (sufficient to offset the expected warming over the next century) to an altitude of 20 to 30 km is estimated at US$2 billion to 8 billion.[109] In comparison, the annual cost estimates for climate damage or emission mitigation range from US$200 billion to 2 trillion.[109]
A 2016 study finds the cost per 1 W/m2 of cooling to be between 5–50 billion USD/yr.[110] Because larger particles are less efficient at cooling and drop out of the sky faster, the unit-cooling cost is expected to increase over time as increased dose leads to larger, but less efficient, particles by mechanism such as coalescence and Ostwald ripening.[111] Assume RCP8.5, -5.5 W/m2 of cooling would be required by 2100 to maintain 2020 climate. At the dose level required to provide this cooling, the net efficiency per mass of injected aerosols would reduce to below 50% compared to low-level deployment (below 1W/m2).[112] At a total dose of -5.5 W/m2, the cost would be between 55-550 billion USD/yr when efficiency reduction is also taken into account, bringing annual expenditure to levels comparable to other mitigation alternatives.
Other possible side effects
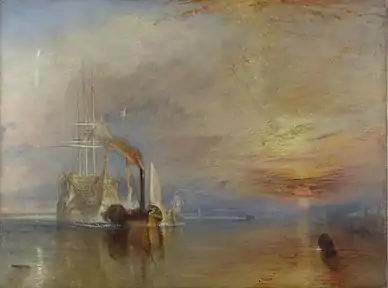
Solar geoengineering in general poses various problems and risks. However, certain problems are specific to or more pronounced with stratospheric sulfide injection.[113]
- Ozone depletion: a potential side effect of sulfur aerosols;[114][115] and these concerns have been supported by modelling.[116] However, this may only occur if high enough quantities of aerosols drift to, or are deposited in, polar stratospheric clouds before the levels of CFCs and other ozone destroying gases fall naturally to safe levels because stratospheric aerosols, together with the ozone destroying gases, are responsible for ozone depletion.[117][118] The injection of other aerosols that may be safer such as calcite has therefore been proposed.[8] The injection of non-sulfide aerosols like calcite (limestone) would also have a cooling effect while counteracting ozone depletion and would be expected to reduce other side effects.[8]
- Whitening of the sky: Volcanic eruptions are known to affect the appearance of sunsets significantly,[119] and a change in sky appearance after the eruption of Mount Tambora in 1816 "The Year Without A Summer" was the inspiration for the paintings of J. M. W. Turner. Since stratospheric aerosol injection would involve smaller quantities of aerosols, it is expected to cause a subtler change to sunsets and a slight hazing of blue skies.[120][121] How stratospheric aerosol injection may affect clouds remains uncertain.[122]
- Stratospheric temperature change: Aerosols can also absorb some radiation from the Sun, the Earth, and the surrounding atmosphere. This changes the surrounding air temperature and could potentially impact the stratospheric circulation, which in turn may impact the surface circulation.[123]
- Deposition and acid rain: The surface deposition of sulfate injected into the stratosphere may also have an impact on ecosystems. However, the amount and wide dispersal of injected aerosols means that their impact on particulate concentrations and acidity of precipitation would be very small.[66]
- Ecological consequences: The consequences of stratospheric aerosol injection on ecological systems are unknown and potentially vary by ecosystem with differing impacts on marine versus terrestrial biomes.[124][125][126]
- Mixed effects on agriculture: A historical study in 2018 found that stratospheric sulfate aerosols injected by the volcanic eruptions of Chicón (1982) and Mount Pinatubo (1991) had mixed effects on global crop yields of certain major crops.[127] Based on several studies, the IPCC Sixth Assessment Report suggests that crop yields and carbon sinks would be largely unaffected or may even increase slightly, because reduced photosynthesis due to lower sunlight would be offset by CO2 fertilization effect and the reduction in thermal stress, but there's less confidence about how the specific ecosystems may be affected.[68]
- Inhibition of Solar Energy Technologies: Uniformly reduced net shortwave radiation would hurt solar photovoltaics by the same 2-5% as for plants.[128] the increased scattering of collimated incoming sunlight would more drastically reduce the efficiencies (by 11% for RCP8.5) of concentrating solar thermal power for both electricity production [129][128] and chemical reactions, such as solar cement production.[130]
Outdoors research
Almost all work to date on stratospheric sulfate injection has been limited to modeling and laboratory work. In 2009, a Russian team tested aerosol formation in the lower troposphere using helicopters.[131] In 2015, David Keith and Gernot Wagner described a potential field experiment, the Stratospheric Controlled Perturbation Experiment (SCoPEx), using stratospheric calcium carbonate[132] injection,[133] but as of October 2020 the time and place had not yet been determined.[134][135] SCoPEx is in part funded by Bill Gates.[136][137] Sir David King, a former chief scientific adviser to the government of the United Kingdom, stated that SCoPEX and Gates' plans to dim the sun with calcium carbonate could have disastrous effects.[138]
In 2012, the Bristol University-led Stratospheric Particle Injection for Climate Engineering (SPICE) project planned on a limited field test in order to evaluate a potential delivery system. The group received support from the EPSRC, NERC and STFC to the tune of £2.1 million[139] and was one of the first UK projects aimed at providing evidence-based knowledge about solar radiation management.[139] Although the field testing was cancelled, the project panel decided to continue the lab-based elements of the project.[140] Furthermore, a consultation exercise was undertaken with members of the public in a parallel project by Cardiff University, with specific exploration of attitudes to the SPICE test.[141] This research found that almost all of the participants in the poll were willing to allow the field trial to proceed, but very few were comfortable with the actual use of stratospheric aerosols. A campaign opposing geoengineering led by the ETC Group drafted an open letter calling for the project to be suspended until international agreement is reached,[142] specifically pointing to the upcoming convention of parties to the Convention on Biological Diversity in 2012.[143]
Governance
Most of the existing governance of stratospheric sulfate aerosols is from that which is applicable to solar radiation management more broadly. However, some existing legal instruments would be relevant to stratospheric sulfate aerosols specifically. At the international level, the Convention on Long-Range Transboundary Air Pollution (CLRTAP Convention) obligates those countries which have ratified it to reduce their emissions of particular transboundary air pollutants. Notably, both solar radiation management and climate change (as well as greenhouse gases) could satisfy the definition of "air pollution" which the signatories commit to reduce, depending on their actual negative effects.[144] Commitments to specific values of the pollutants, including sulfates, are made through protocols to the CLRTAP Convention. Full implementation or large scale climate response field tests of stratospheric sulfate aerosols could cause countries to exceed their limits. However, because stratospheric injections would be spread across the globe instead of concentrated in a few nearby countries, and could lead to net reductions in the "air pollution" which the CLRTAP Convention is to reduce.
The stratospheric injection of sulfate aerosols would cause the Vienna Convention for the Protection of the Ozone Layer to be applicable due to their possible deleterious effects on stratospheric ozone. That treaty generally obligates its Parties to enact policies to control activities which "have or are likely to have adverse effects resulting from modification or likely modification of the ozone layer."[145] The Montreal Protocol to the Vienna Convention prohibits the production of certain ozone depleting substances, via phase outs. Sulfates are presently not among the prohibited substances.
In the United States, the Clean Air Act might give the United States Environmental Protection Agency authority to regulate stratospheric sulfate aerosols.[146]
Welsbach seeding
Welsbach seeding is a patented climate engineering method, involving seeding the stratosphere with small (10 to 100 micron) metal oxide particles (thorium dioxide, aluminium oxide). The purpose of the Welsbach seeding would be to "(reduce) atmospheric warming due to the greenhouse effect resulting from a greenhouse gases layer," by converting radiative energy at near-infrared wavelengths into radiation at far-infrared wavelengths, permitting some of the converted radiation to escape into space, thus cooling the atmosphere. The seeding as described would be performed by airplanes at altitudes between 7 and 13 kilometres.
Patent
The method was patented by Hughes Aircraft Company in 1991, US patent 5003186.[147] Quote from the patent:
"Global warming has been a great concern of many environmental scientists. Scientists believe that the greenhouse effect is responsible for global warming. Greatly increased amounts of heat-trapping gases have been generated since the Industrial Revolution. These gases, such as CO2, CFC, and methane, accumulate in the atmosphere and allow sunlight to stream in freely but block heat from escaping (greenhouse effect). These gases are relatively transparent to sunshine but absorb strongly the long-wavelength infrared radiation released by the earth."
"This invention relates to a method for the reduction of global warming resulting from the greenhouse effect, and in particular to a method which involves the seeding of the earth's stratosphere with Welsbach-like materials."
Feasibility
The method has never been implemented, and is not considered to be a viable option by current geoengineering experts; in fact the proposed mechanism is considered to violate the second law of thermodynamics.[148] Currently proposed atmospheric geoengineering methods would instead use other aerosols, at considerably higher altitudes.[149]
History
Mikhail Budyko is believed to have been the first, in 1974, to put forth the concept of artificial solar radiation management with stratospheric sulfate aerosols if global warming ever became a pressing issue.[150] Such controversial climate engineering proposals for global dimming have sometimes been called a "Budyko Blanket".[151][152][153]
In popular-culture
In the film Snowpiercer, as well as in the television spin-off, an apocalyptic global ice-age is caused by the introduction of a fictional substance, dubbed, CW-7 into the atmosphere, with the intention of preventing global-warming by blocking out the light of the sun. [154][155]
In the Novel The Ministry for the Future by Kim Stanley Robinson, stratospheric aerosol injection is used by the Indian Government as a climate mitigation measure following a catastrophic and deadly heatwave.[156]
See also
- Carl Auer von Welsbach – Austrian scientist and inventor (1858–1929)
- Chemtrail conspiracy theory – Conspiracy theory about contrails
- Climate change – Current rise in Earth's average temperature and its effects
- Climate change mitigation – Actions to reduce net greenhouse gas emissions to limit climate change
- Climate engineering – Deliberate and large-scale intervention in the Earth’s climate system
- Cloud seeding – Method that condenses clouds to cause rainfall
- Global dimming – Reduction in the amount of sunlight reaching Earth's surface
- Solar geoengineering – Reflection of sunlight to reduce global warming
- Termination Shock – Science fiction novel by Neal Stephenson
- Weather Modification Operations and Research Board – Act of intentionally altering or manipulating the weather
References
- Crutzen, P. J. (2006). "Albedo Enhancement by Stratospheric Sulfur Injections: A Contribution to Resolve a Policy Dilemma?". Climatic Change. 77 (3–4): 211–220. Bibcode:2006ClCh...77..211C. doi:10.1007/s10584-006-9101-y.
- Climate Intervention: Reflecting Sunlight to Cool Earth. Washington, D.C.: National Academies Press. 2015-06-23. doi:10.17226/18988. ISBN 9780309314824. Archived from the original on 2021-11-22. Retrieved 2015-11-18.
- Intergovernmental Panel on Climate Change (2018). Global warming of 1.5°C. [Geneva, Switzerland]. p. 350. ISBN 9789291691517. OCLC 1056192590.
{{cite book}}: CS1 maint: location missing publisher (link) - Cziczo, Daniel J.; Wolf, Martin J.; Gasparini, Blaž; Münch, Steffen; Lohmann, Ulrike (2019-12-11). "Unanticipated Side Effects of Stratospheric Albedo Modification Proposals Due to Aerosol Composition and Phase". Scientific Reports. 9 (1): 18825. Bibcode:2019NatSR...918825C. doi:10.1038/s41598-019-53595-3. ISSN 2045-2322. PMC 6906325. PMID 31827104.
- Daisy Dunne (11 March 2019). "Halving global warming with solar geoengineering could 'offset tropical storm risk'". CarbonBrief. Archived from the original on 26 March 2019. Retrieved 14 March 2019.
- Rasch, Philip J; Tilmes, Simone; Turco, Richard P; Robock, Alan; Oman, Luke; Chen, Chih-Chieh (Jack); Stenchikov, Georgiy L; Garcia, Rolando R (29 August 2008). "An overview of geoengineering of climate using stratospheric sulphate aerosols". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 366 (1882): 4007–4037. Bibcode:2008RSPTA.366.4007R. doi:10.1098/rsta.2008.0131. PMID 18757276. S2CID 9869660.
- Tollefson, Jeff (2021-03-29). "US urged to invest in sun-dimming studies as climate warms". Nature. doi:10.1038/d41586-021-00822-5. PMID 33785925. S2CID 232431313. Archived from the original on 2021-08-25. Retrieved 2021-08-25.
- Keith, David W.; Weisenstein, Debra K.; Dykema, John A.; Keutsch, Frank N. (27 December 2016). "Stratospheric solar geoengineering without ozone loss". Proceedings of the National Academy of Sciences. 113 (52): 14910–14914. Bibcode:2016PNAS..11314910K. doi:10.1073/pnas.1615572113. PMC 5206531. PMID 27956628.
- Voosen, Paul (2018-03-21). "A dusting of salt could cool the planet". Science | AAAS. Archived from the original on 2021-08-25. Retrieved 2021-08-25.
- Pierce, J. R.; Weisenstein, D. K.; Heckendorn, P.; Peter, T.; Keith, D. W. (2010). "Efficient formation of stratospheric aerosol for climate engineering by emission of condensible vapor from aircraft". Geophysical Research Letters. 37 (18): n/a. Bibcode:2010GeoRL..3718805P. doi:10.1029/2010GL043975. S2CID 15934540.
- Riva, Matthieu; Chen, Yuzhi; Zhang, Yue; Lei, Ziying; Olson, Nicole E.; Boyer, Hallie C.; Narayan, Shweta; Yee, Lindsay D.; Green, Hilary S.; Cui, Tianqu; Zhang, Zhenfa; Baumann, Karsten; Fort, Mike; Edgerton, Eric; Budisulistiorini, Sri H. (2019-08-06). "Increasing Isoprene Epoxydiol-to-Inorganic Sulfate Aerosol Ratio Results in Extensive Conversion of Inorganic Sulfate to Organosulfur Forms: Implications for Aerosol Physicochemical Properties". Environmental Science & Technology. 53 (15): 8682–8694. Bibcode:2019EnST...53.8682R. doi:10.1021/acs.est.9b01019. ISSN 0013-936X. PMC 6823602. PMID 31335134.
- Allen, Bob (2015-04-06). "Atmospheric Aerosols: What Are They, and Why Are They So Important?". NASA. Retrieved 2023-04-17.
- Cai, Zhixiong; Li, Feiming; Rong, Mingcong; Lin, Liping; Yao, Qiuhong; Huang, Yipeng; Chen, Xi; Wang, Xiaoru (2019-01-01), Wang, Xiaoru; Chen, Xi (eds.), "Chapter 1 - Introduction", Novel Nanomaterials for Biomedical, Environmental and Energy Applications, Micro and Nano Technologies, Elsevier, pp. 1–36, ISBN 978-0-12-814497-8, retrieved 2023-04-19
- Legras, Bernard; Duchamp, Clair; Sellitto, Pasquale; Podglajen, Aurélien; Carboni, Elisa; Siddans, Richard; Grooß, Jens-Uwe; Khaykin, Sergey; Ploeger, Felix (23 November 2022). "The evolution and dynamics of the Hunga Tonga plume in the stratosphere". Atmospheric Chemistry and Physics. 22 (22): 14957–14970. doi:10.5194/acp-22-14957-2022. S2CID 253875202.
- "Glossary". earthobservatory.nasa.gov. 2023-04-18. Retrieved 2023-04-18.
- Seinfeld, John H.; Pandis, Spyros N (1998). Atmospheric Chemistry and Physics — From Air Pollution to Climate Change. John Wiley and Sons, Inc. ISBN 978-0-471-17816-3
- Charlson, Robert J.; Wigley, Tom M. L. (1994). "Sulfate Aerosol and Climatic Change". Scientific American. 270 (2): 48–57. Bibcode:1994SciAm.270b..48C. doi:10.1038/scientificamerican0294-48. ISSN 0036-8733. JSTOR 24942590.
- Keith, D. W. (7 September 2010). "Photophoretic levitation of engineered aerosols for geoengineering". Proceedings of the National Academy of Sciences. 107 (38): 16428–16431. Bibcode:2010PNAS..10716428K. doi:10.1073/pnas.1009519107. PMC 2944714. PMID 20823254.
- "Volcanic Sulfur Aerosols Affect Climate and the Earth's Ozone Layer". United States Geological Survey. Retrieved 17 February 2009.
- Mathera, T.A., C. Oppenheimer, A.G. Allen and A.J.S. McGonigle (2004). "Aerosol chemistry of emissions from three contrasting volcanoes in Italy". Atmospheric Environment. 38 (33): 5637–5649. Bibcode:2004AtmEn..38.5637M. doi:10.1016/j.atmosenv.2004.06.017.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - IPCC, 1990: Chapter 1: Greenhouse Gases and Aerosols [R.T. Watson, H. Rodhe, H. Oeschger and U. Siegenthaler]. In: Climate Change: The IPCC Scientific Assessment [J.T.Houghton, G.J.Jenkins and J.J.Ephraums (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 31–34,
- Effects of Acid Rain – Human Health Archived January 18, 2008, at the Wayback Machine. Epa.gov (June 2, 2006). Retrieved on 2013-02-09.
- Bates, T. S.; Lamb, B. K.; Guenther, A.; Dignon, J.; Stoiber, R. E. (April 1992). "Sulfur emissions to the atmosphere from natural sources". Journal of Atmospheric Chemistry. 14 (1–4): 315–337. Bibcode:1992JAtC...14..315B. doi:10.1007/BF00115242. ISSN 0167-7764. S2CID 55497518.
- Burns, Douglas A.; Aherne, Julian; Gay, David A.; Lehmann, Christopher M.~B. (2016). "Acid rain and its environmental effects: Recent scientific advances". Atmospheric Environment. 146: 1–4. Bibcode:2016AtmEn.146....1B. doi:10.1016/j.atmosenv.2016.10.019.
- "Effects of Acid Rain - Surface Waters and Aquatic Animals". US EPA. Archived from the original on 14 May 2009.
- Rodhe, Henning; Dentener, Frank; Schulz, Michael (2002-10-01). "The Global Distribution of Acidifying Wet Deposition". Environmental Science & Technology. 36 (20): 4382–4388. Bibcode:2002EnST...36.4382R. doi:10.1021/es020057g. ISSN 0013-936X. PMID 12387412.
- US EPA: Effects of Acid Rain – Forests Archived July 26, 2008, at the Wayback Machine
- Likens, G. E.; Driscoll, C. T.; Buso, D. C. (1996). "Long-Term Effects of Acid Rain: Response and Recovery of a Forest Ecosystem" (PDF). Science. 272 (5259): 244. Bibcode:1996Sci...272..244L. doi:10.1126/science.272.5259.244. S2CID 178546205. Archived (PDF) from the original on December 24, 2012. Retrieved February 9, 2013.
- Larssen, T.; Carmichael, G. R. (2000-10-01). "Acid rain and acidification in China: the importance of base cation deposition". Environmental Pollution. 110 (1): 89–102. doi:10.1016/S0269-7491(99)00279-1. ISSN 0269-7491. PMID 15092859. Archived from the original on March 30, 2015. Retrieved April 22, 2020.
- Johnson, Dale W.; Turner, John; Kelly, J. M. (1982). "The effects of acid rain on forest nutrient status". Water Resources Research. 18 (3): 449–461. Bibcode:1982WRR....18..449J. doi:10.1029/WR018i003p00449. ISSN 1944-7973.
- Wang, X.; Ding, H.; Ryan, L.; Xu, X. (1 May 1997). "Association between air pollution and low birth weight: a community-based study". Environmental Health Perspectives. 105 (5): 514–20. doi:10.1289/ehp.97105514. ISSN 0091-6765. PMC 1469882. PMID 9222137. S2CID 2707126.
- Lin, Cheng-Kuan; Lin, Ro-Ting; Chen, Pi-Cheng; Wang, Pu; De Marcellis-Warin, Nathalie; Zigler, Corwin; Christiani, David C. (2018-02-08). "A Global Perspective on Sulfur Oxide Controls in Coal-Fired Power Plants and Cardiovascular Disease". Scientific Reports. 8 (1): 2611. Bibcode:2018NatSR...8.2611L. doi:10.1038/s41598-018-20404-2. ISSN 2045-2322. PMC 5805744. PMID 29422539.
- Lin, Cheng-Kuan; Lin, Ro-Ting; Chen, Pi-Cheng; Wang, Pu; De Marcellis-Warin, Nathalie; Zigler, Corwin; Christiani, David C. (2018-02-08). "A Global Perspective on Sulfur Oxide Controls in Coal-Fired Power Plants and Cardiovascular Disease". Scientific Reports. 8 (1): 2611. Bibcode:2018NatSR...8.2611L. doi:10.1038/s41598-018-20404-2. ISSN 2045-2322. PMC 5805744. PMID 29422539.
- Clean Air Act Reduces Acid Rain In Eastern United States Archived August 8, 2018, at the Wayback Machine, ScienceDaily, September 28, 1998
- "Air Emissions Trends – Continued Progress Through 2005". U.S. Environmental Protection Agency. 8 July 2014. Archived from the original on 2007-03-17. Retrieved 2007-03-17.
- Effects of Acid Rain – Human Health Archived January 18, 2008, at the Wayback Machine. Epa.gov (June 2, 2006). Retrieved on 2013-02-09.
- Carrington, Damian (2021-09-06). "More global aid goes to fossil fuel projects than tackling dirty air – study". The Guardian. Retrieved 2021-09-07.
- Moses, Elizabeth; Cardenas, Beatriz; Seddon, Jessica (25 February 2020). "The Most Successful Air Pollution Treaty You've Never Heard Of".
- China has its worst spell of acid rain, United Press International (2006-09-22).
- He, Yanyi; Wang, Kaicun; Zhou, Chunlüe; Wild, Martin (15 April 2022). "Evaluation of surface solar radiation trends over China since the 1960s in the CMIP6 models and potential impact of aerosol emissions". Atmospheric Research. 268: 105991. Bibcode:2022AtmRe.26805991W. doi:10.1016/j.atmosres.2021.105991. S2CID 245483347.
- Kuttippurath, J.; Patel, V. K.; Pathak, M.; Singh, A. (2022). "Improvements in SO2 pollution in India: role of technology and environmental regulations". Environmental Science and Pollution Research. 29 (52): 78637–78649. doi:10.1007/s11356-022-21319-2. ISSN 1614-7499. PMC 9189448. PMID 35696063. S2CID 249613744.
- Baroni, M., M.H. Thiemens, R.J. Delmas, and J. Savarino (2007). "Mass-Independent Sulfur Isotopic Compositions in Stratospheric Volcanic Eruptions". Science. 315 (5808): 84–87. Bibcode:2007Sci...315...84B. doi:10.1126/science.1131754. PMID 17204647. S2CID 40342760.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Self, S., J.-X. Zhao, R.E. Holasek, R.C. Torres, and A.J. King (1997). "The Atmospheric Impact of the 1991 Mount Pinatubo Eruption". Fire and Mud: Eruptions and Lahars of Mount Pinatubo, Philippines. University of Washington Press. ISBN 978-0-295-97585-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Jason Wolfe (5 September 2000). "Volcanoes and Climate Change". Earth Observatory. NASA. Retrieved 19 February 2009.
- Robock, A. (2008). "20 reasons why geoengineering may be a bad idea" (PDF). Bulletin of the Atomic Scientists. 64 (2): 14–18. Bibcode:2008BuAtS..64b..14R. doi:10.2968/064002006.
- Rampino MR, Self S (23 August 1984). "Sulphur-rich volcanic eruptions and stratospheric aerosols". Nature. 310 (5979): 677–9. Bibcode:1984Natur.310..677R. doi:10.1038/310677a0. S2CID 4332484.
- H. Gilgen; M. Wild; A. Ohmura (1998). "Means and trends of shortwave irradiance at the surface estimated from global energy balance archive data" (PDF). Journal of Climate. 11 (8): 2042–2061. Bibcode:1998JCli...11.2042G. doi:10.1175/1520-0442-11.8.2042.
- Stanhill, G.; S. Cohen (2001). "Global dimming: a review of the evidence for a widespread and significant reduction in global radiation with discussion of its probable causes and possible agricultural consequences". Agricultural and Forest Meteorology. 107 (4): 255–278. Bibcode:2001AgFM..107..255S. doi:10.1016/S0168-1923(00)00241-0.
- Liepert, B. G. (2 May 2002). "Observed Reductions in Surface Solar Radiation in the United States and Worldwide from 1961 to 1990" (PDF). Geophysical Research Letters. 29 (12): 61–1–61–4. Bibcode:2002GeoRL..29.1421L. doi:10.1029/2002GL014910.
- Eddy, John A.; Gilliland, Ronald L.; Hoyt, Douglas V. (23 December 1982). "Changes in the solar constant and climatic effects". Nature. 300 (5894): 689–693. Bibcode:1982Natur.300..689E. doi:10.1038/300689a0. S2CID 4320853.
Spacecraft measurements have established that the total radiative output of the Sun varies at the 0.1−0.3% level
- Cohen, Shabtai; Stanhill, Gerald (1 January 2021), Letcher, Trevor M. (ed.), "Chapter 32 – Changes in the Sun's radiation: the role of widespread surface solar radiation trends in climate change: dimming and brightening", Climate Change (Third Edition), Elsevier, pp. 687–709, doi:10.1016/b978-0-12-821575-3.00032-3, ISBN 978-0-12-821575-3, S2CID 234180702, retrieved 2023-04-26
- "Global 'Sunscreen' Has Likely Thinned, Report NASA Scientists". NASA. 15 March 2007. Archived from the original on 22 December 2018. Retrieved 28 June 2023.
- "A bright sun today? It's down to the atmosphere". The Guardian. 2017. Archived from the original on 2017-05-20. Retrieved 2017-05-19.
- Seneviratne, S.I.; Zhang, X.; Adnan, M.; Badi, W.; Dereczynski, C.; Di Luca, A.; Ghosh, S.; Iskandar, I.; Kossin, J.; Lewis, S.; Otto, F.; Pinto, I.; Satoh, M.; Vicente-Serrano, S. M.; Wehner, M.; Zhou, B. (2021). Masson-Delmotte, V.; Zhai, P.; Piran, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L. (eds.). "Weather and Climate Extreme Events in a Changing Climate" (PDF). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. 2021: 1238. Bibcode:2021AGUFM.U13B..05K. doi:10.1017/9781009157896.007.
- Wild, M.; Ohmura, A.; Makowski, K. (2007). "Impact of global dimming and brightening on global warming". Geophysical Research Letters. 34 (4): L04702. Bibcode:2007GeoRL..34.4702W. doi:10.1029/2006GL028031.
- ""Warming Hole" Over the Eastern U.S. Due to Air Pollution". NASA. 18 May 2012.
- Karmalkar, Ambarish V.; Horton, Radley M. (23 September 2021). "Drivers of exceptional coastal warming in the northeastern United States". Nature Climate Change. 11 (10): 854–860. Bibcode:2021NatCC..11..854K. doi:10.1038/s41558-021-01159-7. S2CID 237611075.
- Krajick, Kevin (23 September 2021). "Why the U.S. Northeast Coast Is a Global Warming Hot Spot". Columbia Climate School. Retrieved 2023-03-23.
- Kobayashi, Yuya; Ide, Yu; Takegawa, Nobuyuki (2021-04-03). "Development of a novel particle mass spectrometer for online measurements of refractory sulfate aerosols". Aerosol Science and Technology. 55 (4): 371–386. Bibcode:2021AerST..55..371K. doi:10.1080/02786826.2020.1852168. ISSN 0278-6826. S2CID 229506768.
- Palumbo, P., A. Rotundi, V. Della Corte, A. Ciucci, L. Colangeli, F. Esposito, E. Mazzotta Epifani, V. Mennella , J.R. Brucato, F.J.M. Rietmeijer, G. J. Flynn, J.-B. Renard, J.R. Stephens, and E. Zona. "The DUSTER experiment: collection and analysis of aerosol in the high stratosphere". Retrieved 19 February 2009.
{{cite web}}: CS1 maint: multiple names: authors list (link) - Myhre, Gunnar; Stordal, Frode; Berglen, Tore F.; Sundet, Jostein K.; Isaksen, Ivar S. A. (2004-03-01). "Uncertainties in the Radiative Forcing Due to Sulfate Aerosols". Journal of the Atmospheric Sciences. 61 (5): 485–498. Bibcode:2004JAtS...61..485M. doi:10.1175/1520-0469(2004)061<0485:UITRFD>2.0.CO;2. ISSN 0022-4928. S2CID 55623817.
- Zhang, Jie; Furtado, Kalli; Turnock, Steven T.; Mulcahy, Jane P.; Wilcox, Laura J.; Booth, Ben B.; Sexton, David; Wu, Tongwen; Zhang, Fang; Liu, Qianxia (22 December 2021). "The role of anthropogenic aerosols in the anomalous cooling from 1960 to 1990 in the CMIP6 Earth system models". Atmospheric Chemistry and Physics. 21 (4): 18609–18627. Bibcode:2021ACP....2118609Z. doi:10.5194/acp-21-18609-2021.
- "Aerosols and Incoming Sunlight (Direct Effects)". NASA. 2 November 2010.
- "Stratospheric Injections Could Help Cool Earth, Computer Model Shows". ScienceDaily. 15 September 2006. Retrieved 19 February 2009.
- Launder B. & J.M.T. Thompson (1996). "Global and Arctic climate engineering: numerical model studies". Phil. Trans. R. Soc. A. 366 (1882): 4039–56. Bibcode:2008RSPTA.366.4039C. doi:10.1098/rsta.2008.0132. PMID 18757275.
- Visioni, Daniele; Slessarev, Eric; MacMartin, Douglas G; Mahowald, Natalie M; Goodale, Christine L; Xia, Lili (1 September 2020). "What goes up must come down: impacts of deposition in a sulfate geoengineering scenario". Environmental Research Letters. 15 (9): 094063. Bibcode:2020ERL....15i4063V. doi:10.1088/1748-9326/ab94eb. ISSN 1748-9326.
- Andrew Charlton-Perez & Eleanor Highwood. "Costs and benefits of geo-engineering in the Stratosphere" (PDF). Archived from the original (PDF) on 14 January 2017. Retrieved 17 February 2009.
- Trisos, Christopher H.; Geden, Oliver; Seneviratne, Sonia I.; Sugiyama, Masahiro; van Aalst, Maarten; Bala, Govindasamy; Mach, Katharine J.; Ginzburg, Veronika; de Coninck, Heleen; Patt, Anthony (2021). "Cross-Working Group Box SRM: Solar Radiation Modification" (PDF). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. 2021: 1238. Bibcode:2021AGUFM.U13B..05K. doi:10.1017/9781009157896.007.
- Victor, David G.; Morgan, M. Granger; Apt, Jay; Steinbruner, John; Ricke, Katharine (March–April 2009). "The Geoengineering Option:A Last Resort Against Global Warming?". Geoengineering. Council on Foreign Affairs. Archived from the original on April 21, 2010. Retrieved August 19, 2009.
- Wigley, T. M. L. (20 October 2006). "A Combined Mitigation/Geoengineering Approach to Climate Stabilization". Science. 314 (5798): 452–454. Bibcode:2006Sci...314..452W. doi:10.1126/science.1131728. PMID 16973840. S2CID 40846810. Archived from the original on 12 August 2019. Retrieved 1 July 2019.
- "Stratospheric Injections Could Help Cool Earth, Computer Model Shows – News Release". National Center for Atmospheric Research. September 14, 2006. Archived from the original on May 8, 2017. Retrieved June 15, 2011.
- Keith, D.W. and D. K. Weisenstein (2015). "Solar geoengineering using solid aerosol in the stratosphere". Atmos. Chem. Phys. Discuss. 15 (8): 11799–11851. Bibcode:2015ACP....1511835W. doi:10.5194/acpd-15-11799-2015.
- Ferraro, A. J.; Charlton-Perez, A. J.; Highwood, E. J. (27 January 2015). "Stratospheric dynamics and midlatitude jets under geoengineering with space mirrors and sulfate and titania aerosols" (PDF). Journal of Geophysical Research: Atmospheres. 120 (2): 414–429. Bibcode:2015JGRD..120..414F. doi:10.1002/2014JD022734. hdl:10871/16214. S2CID 33804616. Archived (PDF) from the original on 28 April 2019. Retrieved 1 July 2019.
- McClellan, Justin; Keith, David; Apt, Jay (30 August 2012). "Cost Analysis of Stratospheric Albedo Modification Delivery Systems". Environmental Research Letters. 7 (3): 3 in 1–8. doi:10.1088/1748-9326/7/3/034019.
- Smith, Wake; Wagner, Gernot (2018). "Stratospheric aerosol injection tactics and costs in the first 15 years of deployment". Environmental Research Letters. 13 (12): 124001. Bibcode:2018ERL....13l4001S. doi:10.1088/1748-9326/aae98d.
- Robock, A.; Marquardt, A.; Kravitz, B.; Stenchikov, G. (2009). "Benefits, risks, and costs of stratospheric geoengineering". Geophysical Research Letters. 36 (19): L19703. Bibcode:2009GeoRL..3619703R. doi:10.1029/2009GL039209. hdl:10754/552099. S2CID 34488313.
- PICATINNY ARSENAL DOVER N J. "PARAMETRIC STUDIES ON USE OF BOOSTED ARTILLERY PROJECTILES FOR HIGH ALTITUDE RESEARCH PROBES, PROJECT HARP". Archived from the original on January 14, 2017. Retrieved February 25, 2009.
- English, J. M.; Toon, O. B.; Mills, M. J. (2012). "Microphysical simulations of sulfur burdens from stratospheric sulfur geoengineering". Atmospheric Chemistry and Physics. 12 (10): 4775–4793. Bibcode:2012ACP....12.4775E. doi:10.5194/acp-12-4775-2012.
- MacCracken, M. C.; Shin, H. -J.; Caldeira, K.; Ban-Weiss, G. A. (2012). "Climate response to imposed solar radiation reductions in high latitudes". Earth System Dynamics Discussions. 3 (2): 715–757. Bibcode:2013ESD.....4..301M. doi:10.5194/esdd-3-715-2012.
- Niemeier, U.; Schmidt, H.; Timmreck, C. (2011). "The dependency of geoengineered sulfate aerosol on the emission strategy". Atmospheric Science Letters. 12 (2): 189–194. Bibcode:2011AtScL..12..189N. doi:10.1002/asl.304. hdl:11858/00-001M-0000-0011-F582-9. S2CID 120005838. Archived from the original on 2021-08-18. Retrieved 2019-12-07.
- Brovkin, V.; Petoukhov, V.; Claussen, M.; Bauer, E.; Archer, D.; Jaeger, C. (2008). "Geoengineering climate by stratospheric sulfur injections: Earth system vulnerability to technological failure". Climatic Change. 92 (3–4): 243–259. doi:10.1007/s10584-008-9490-1. Archived from the original on 2020-12-06. Retrieved 2019-09-05.
- Smith, Wake (October 2020). "The cost of stratospheric aerosol injection through 2100". Environmental Research Letters. 15 (11): 114004. Bibcode:2020ERL....15k4004S. doi:10.1088/1748-9326/aba7e7. ISSN 1748-9326. S2CID 225534263.
- Bates, S. S.; Lamb, B. K.; Guenther, A.; Dignon, J.; Stoiber, R. E. (1992). "Sulfur emissions to the atmosphere from natural sources". Journal of Atmospheric Chemistry. 14 (1–4): 315–337. Bibcode:1992JAtC...14..315B. doi:10.1007/BF00115242. S2CID 55497518. Archived from the original on 2020-06-19. Retrieved 2019-12-07.
- Zhao, J.; Turco, R. P.; Toon, O. B. (1995). "A model simulation of Pinatubo volcanic aerosols in the stratosphere". Journal of Geophysical Research. 100 (D4): 7315–7328. Bibcode:1995JGR...100.7315Z. doi:10.1029/94JD03325. hdl:2060/19980018652.
- Lenton, Tim; Vaughan. "Radiative forcing potential of climate geoengineering" (PDF). Archived (PDF) from the original on February 26, 2009. Retrieved February 28, 2009.
- Matthews, H. D.; Caldeira, K. (Jun 2007). "Transient climate–carbon simulations of planetary geoengineering". Proceedings of the National Academy of Sciences of the United States of America. 104 (24): 9949–9954. Bibcode:2007PNAS..104.9949M. doi:10.1073/pnas.0700419104. ISSN 0027-8424. PMC 1885819. PMID 17548822.
- Monastersky, Richard (1992). "Haze clouds the greenhouse—sulfur pollution slows global warming—includes related article". Science News.
- Rasch, P. J.; Crutzen, P. J.; Coleman, D. B. (2008). "Exploring the geoengineering of climate using stratospheric sulfate aerosols: the role of particle size". Geophysical Research Letters. 35 (2): L02809. doi:10.1029/2007GL032179. Archived from the original on 2017-10-30. Retrieved 2017-10-29.
- Pierce, Jeffrey R.; Weisenstein, Debra K.; Heckendorn, Patricia; Peter, Thomas; Keith, David W. (September 2010). "Efficient formation of stratospheric aerosol for climate engineering by emission of condensible vapor from aircraft". Geophysical Research Letters. 37 (18): n/a. Bibcode:2010GeoRL..3718805P. doi:10.1029/2010GL043975. S2CID 15934540.
- Niemeier, U.; Schmidt, H.; Timmreck, C. (April 2011). "The dependency of geoengineered sulfate aerosol on the emission strategy". Atmospheric Science Letters. 12 (2): 189–194. Bibcode:2011AtScL..12..189N. doi:10.1002/asl.304. hdl:11858/00-001M-0000-0011-F582-9. S2CID 120005838. Archived from the original on 2021-08-18. Retrieved 2019-12-07.
- Niemeier, U.; Timmreck, C. (2015). "ACP – Peer review – What is the limit of climate engineering by stratospheric injection of SO2?". Atmospheric Chemistry and Physics. 15 (16): 9129–9141. Bibcode:2015ACP....15.9129N. doi:10.5194/acp-15-9129-2015.
- Gillett, Nathan P.; Kirchmeier-Young, Megan; Ribes, Aurélien; Shiogama, Hideo; Hegerl, Gabriele C.; Knutti, Reto; Gastineau, Guillaume; John, Jasmin G.; Li, Lijuan; Nazarenko, Larissa; Rosenbloom, Nan; Seland, Øyvind; Wu, Tongwen; Yukimoto, Seiji; Ziehn, Tilo (18 January 2021). "Constraining human contributions to observed warming since the pre-industrial period" (PDF). Nature Climate Change. 11 (3): 207–212. Bibcode:2021NatCC..11..207G. doi:10.1038/s41558-020-00965-9. S2CID 231670652.
- IPCC, 2021: Summary for Policymakers. In: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 3–32, doi:10.1017/9781009157896.001.
- Andrew, Tawana (27 September 2019). "Behind the Forecast: How clouds affect temperatures". Science Behind the Forecast. LOUISVILLE, Ky. (WAVE). Retrieved 4 January 2023.
- McCoy, Daniel T.; Field, Paul; Gordon, Hamish; Elsaesser, Gregory S.; Grosvenor, Daniel P. (6 April 2020). "Untangling causality in midlatitude aerosol–cloud adjustments". Atmospheric Chemistry and Physics. 20 (7): 4085–4103. Bibcode:2020ACP....20.4085M. doi:10.5194/acp-20-4085-2020.
- Sato, Yousuke; Goto, Daisuke; Michibata, Takuro; Suzuki, Kentaroh; Takemura, Toshihiko; Tomita, Hirofumi; Nakajima, Teruyuki (7 March 2018). "Aerosol effects on cloud water amounts were successfully simulated by a global cloud-system resolving model". Nature Communications. 9 (1): 985. Bibcode:2018NatCo...9..985S. doi:10.1038/s41467-018-03379-6. PMC 5841301. PMID 29515125.
- Rosenfeld, Daniel; Zhu, Yannian; Wang, Minghuai; Zheng, Youtong; Goren, Tom; Yu, Shaocai (2019). "Aerosol-driven droplet concentrations dominate coverage and water of oceanic low level clouds" (PDF). Science. 363 (6427): eaav0566. doi:10.1126/science.aav0566. PMID 30655446. S2CID 58612273.
- Glassmeier, Franziska; Hoffmann, Fabian; Johnson, Jill S.; Yamaguchi, Takanobu; Carslaw, Ken S.; Feingold, Graham (29 January 2021). "Aerosol-cloud-climate cooling overestimated by ship-track data". Science. 371 (6528): 485–489. Bibcode:2021Sci...371..485G. doi:10.1126/science.abd3980. PMID 33510021.
- Manshausen, Peter; Watson-Parris, Duncan; Christensen, Matthew W.; Jalkanen, Jukka-Pekka; Stier, Philip Stier (7 March 2018). "Invisible ship tracks show large cloud sensitivity to aerosol". Nature. 610 (7930): 101–106. doi:10.1038/s41586-022-05122-0. PMC 9534750. PMID 36198778.
- Jongebloed, U. A.; Schauer, A. J.; Cole-Dai, J.; Larrick, C. G.; Wood, R.; Fischer, T. P.; Carn, S. A.; Salimi, S.; Edouard, S. R.; Zhai, S.; Geng, L.; Alexander, B. (2 January 2023). "Underestimated Passive Volcanic Sulfur Degassing Implies Overestimated Anthropogenic Aerosol Forcing". Geophysical Research Letters. 50 (1): e2022GL102061. Bibcode:2023GeoRL..5002061J. doi:10.1029/2022GL102061. S2CID 255571342.
- Xie, Xiaoning; Myhre, Gunnar; Shindell, Drew; Faluvegi, Gregory; Takemura, Toshihiko; Voulgarakis, Apostolos; Shi, Zhengguo; Li, Xinzhou; Xie, Xiaoxun; Liu, Heng; Liu, Xiaodong; Liu, Yangang (27 December 2022). "Anthropogenic sulfate aerosol pollution in South and East Asia induces increased summer precipitation over arid Central Asia". Communications Earth & Environment. 3 (1): 328. Bibcode:2022ComEE...3..328X. doi:10.1038/s43247-022-00660-x. PMC 9792934. PMID 36588543.
- Lau, K. M.; Kim, K. M. (8 November 2006). "Observational relationships between aerosol and Asian monsoon rainfall, and circulation". Geophysical Research Letters. 33 (21). Bibcode:2006GeoRL..3321810L. doi:10.1029/2006GL027546. S2CID 129282371.
- Rotstayn and Lohmann; Lohmann, Ulrike (2002). "Tropical Rainfall Trends and the Indirect Aerosol Effect". Journal of Climate. 15 (15): 2103–2116. Bibcode:2002JCli...15.2103R. doi:10.1175/1520-0442(2002)015<2103:TRTATI>2.0.CO;2. S2CID 55802370.
- "Global Dimming". bbc.co.uk. BBC. Retrieved 2020-01-05.
- Hirasawa, Haruki; Kushner, Paul J.; Sigmond, Michael; Fyfe, John; Deser, Clara (2 May 2022). "Evolving Sahel Rainfall Response to Anthropogenic Aerosols Driven by Shifting Regional Oceanic and Emission Influences". Journal of Climate. 35 (11): 3181–3193. Bibcode:2022JCli...35.3181H. doi:10.1175/JCLI-D-21-0795.1.
- Jiang, Jiu; Cao, Long; MacMartin, Douglas G.; Simpson, Isla R.; Kravitz, Ben; Cheng, Wei; Visioni, Daniele; Tilmes, Simone; Richter, Jadwiga H.; Mills, Michael J. (2019-12-16). "Stratospheric Sulfate Aerosol Geoengineering Could Alter the High‐Latitude Seasonal Cycle". Geophysical Research Letters. 46 (23): 14153–14163. Bibcode:2019GeoRL..4614153J. doi:10.1029/2019GL085758. ISSN 0094-8276. S2CID 214451704.
- Bala, G.; Duffy, B.; Taylor, E. (June 2008). "Impact of geoengineering schemes on the global hydrological cycle". Proceedings of the National Academy of Sciences of the United States of America. 105 (22): 7664–7669. Bibcode:2008PNAS..105.7664B. doi:10.1073/pnas.0711648105. ISSN 0027-8424. PMC 2409412. PMID 18505844.
- McClellan, Justin; Keith, David W; Apt, Jay (1 September 2012). "Cost analysis of stratospheric albedo modification delivery systems". Environmental Research Letters. 7 (3): 034019. doi:10.1088/1748-9326/7/3/034019.
- Moriyama, Ryo; Sugiyama, Masahiro; Kurosawa, Atsushi; Masuda, Kooiti; Tsuzuki, Kazuhiro; Ishimoto, Yuki (2017). "The cost of stratospheric climate engineering revisited". Mitigation and Adaptation Strategies for Global Change. 22 (8): 1207–1228. doi:10.1007/s11027-016-9723-y. S2CID 157441259. Archived from the original on 2021-07-13. Retrieved 2020-10-21.
- Heckendorn, P; Weisenstein, D; Fueglistaler, S; Luo, B P; Rozanov, E; Schraner, M; Thomason, M; Peter, T (2009). "The impact of geoengineering aerosols on stratospheric temperature and ozone". Environ. Res. Lett. 4 (4): 045108. Bibcode:2009ERL.....4d5108H. doi:10.1088/1748-9326/4/4/045108.
- Niemeier, U.; Timmreck, U. (2015). "What is the limit of climate engineering by stratospheric injection of SO2". Atmos. Chem. Phys. 15 (16): 9129–9141. Bibcode:2015ACP....15.9129N. doi:10.5194/acp-15-9129-2015. Archived from the original on 2020-09-27. Retrieved 2020-10-21.
- Robock, A. (2008). "20 reasons why geoengineering may be a bad idea" (PDF). Bulletin of the Atomic Scientists. 64 (2): 14–19. Bibcode:2008BuAtS..64b..14R. doi:10.2968/064002006. S2CID 145468054. Archived from the original (PDF) on 2020-02-07.
- Tabazadeh, A.; Drdla, K.; Schoeberl, M. R.; Hamill, P.; Toon, O. B. (19 February 2002). "Arctic 'ozone hole' in a cold volcanic stratosphere". Proceedings of the National Academy of Sciences. 99 (5): 2609–12. Bibcode:2002PNAS...99.2609T. doi:10.1073/pnas.052518199. PMC 122395. PMID 11854461.
- Kenzelmann, Patricia; Weissenstein, D; Peter, T; Luo, B; Fueglistaler, S; Rozanov, E; Thomason, L (1 February 2009). "Geo-engineering side effects: Heating the tropical tropopause by sedimenting sulphur aerosol?". IOP Conference Series: Earth and Environmental Science. 6 (45): 452017. Bibcode:2009E&ES....6S2017K. doi:10.1088/1755-1307/6/45/452017. S2CID 250687073.
- Heckendorn, P; Weisenstein, D; Fueglistaler, S; Luo, B P; Rozanov, E; Schraner, M; Thomason, L W; Peter, T (2009). "The impact of geoengineering aerosols on stratospheric temperature and ozone". Environmental Research Letters. 4 (4): 045108. Bibcode:2009ERL.....4d5108H. doi:10.1088/1748-9326/4/4/045108.
- Hargreaves, Ben (2010). "Protecting the Planet". Professional Engineering. 23 (19): 18–22. Archived from the original on 2020-07-12. Retrieved 2020-07-11.
- Pitari, Giovanni; Aquila, Valentina; Kravitz, Ben; Robock, Alan; Watanabe, Shingo; Cionni, Irene; Luca, Natalia De; Genova, Glauco Di; Mancini, Eva; Tilmes, Simone (2014-03-16). "Stratospheric ozone response to sulfate geoengineering: Results from the Geoengineering Model Intercomparison Project (GeoMIP): GeoMIP ozone response". Journal of Geophysical Research: Atmospheres. 119 (5): 2629–2653. doi:10.1002/2013JD020566. S2CID 3576605.
- Olson, D. W., R. L. Doescher, and M. S. Olson (February 2004). "When the sky ran red: The story behind The Scream". Vol. 107, no. 2. Sky & Telescope. pp. 29–35.
{{cite news}}: CS1 maint: multiple names: authors list (link) - LaRC, Denise Adams. "NASA – Geoengineering: Why or Why Not?". www.nasa.gov. Archived from the original on 2021-06-09. Retrieved 2021-06-11.
- Kravitz, Ben; MacMartin, Douglas G.; Caldeira, Ken (2012). "Geoengineering: Whiter skies?". Geophysical Research Letters. 39 (11): n/a. Bibcode:2012GeoRL..3911801K. doi:10.1029/2012GL051652. ISSN 1944-8007. S2CID 17850924.
- Visioni, Daniele; MacMartin, Douglas G.; Kravitz, Ben (2021). "Is Turning Down the Sun a Good Proxy for Stratospheric Sulfate Geoengineering?". Journal of Geophysical Research: Atmospheres. 126 (5): e2020JD033952. Bibcode:2021JGRD..12633952V. doi:10.1029/2020JD033952. ISSN 2169-8996. S2CID 233993808. Archived from the original on 2021-06-11. Retrieved 2021-06-11.
- Ferraro, A. J.; Highwood, E. J.; Charlton-Perez, A. J. (2011). "Stratospheric heating by geoengineering aerosols". Geophysical Research Letters. 37 (24): L24706. Bibcode:2011GeoRL..3824706F. doi:10.1029/2011GL049761. hdl:10871/16215. S2CID 55585854.
- Zarnetske, Phoebe L.; Gurevitch, Jessica; Franklin, Janet; Groffman, Peter M.; Harrison, Cheryl S.; Hellmann, Jessica J.; Hoffman, Forrest M.; Kothari, Shan; Robock, Alan; Tilmes, Simone; Visioni, Daniele (2021-04-13). "Potential ecological impacts of climate intervention by reflecting sunlight to cool Earth". Proceedings of the National Academy of Sciences. 118 (15): e1921854118. Bibcode:2021PNAS..11821854Z. doi:10.1073/pnas.1921854118. ISSN 0027-8424. PMC 8053992. PMID 33876741.
- Howell, Elizabeth (April 19, 2021). "Can we reflect sunlight to fight climate change? Scientists eye aerosol shield for Earth". Space.com. Archived from the original on 2021-07-24. Retrieved 2021-07-24.
- Wood, Charlie (12 April 2021). "'Dimming' the sun poses too many unknowns for Earth". Popular Science. Archived from the original on 24 July 2021. Retrieved 24 July 2021.
- Proctor J, Hsiang S, Burney J, Burke M, Schlenker W (August 2018). "Estimating global agricultural effects of geoengineering using volcanic eruptions". Nature. 560 (7719): 480–483. Bibcode:2018Natur.560..480P. doi:10.1038/s41586-018-0417-3. PMID 30089909. S2CID 51939867. Archived from the original on 2021-11-16. Retrieved 2021-11-16.
- Murphy, Daniel (2009). "Effect of Stratospheric Aerosols on Direct Sunlight and Implications for Concentrating Solar Power". Environ. Sci. Technol. 43 (8): 2783–2786. Bibcode:2009EnST...43.2784M. doi:10.1021/es802206b. PMID 19475950. Retrieved 20 October 2020.
- Smith, Christopher J; Crook, Julia A.; Crook, Rolf; Jackson, Lawrence S.; Osprey, Scott M.; Forster, Piers M. (2017). "Impacts of Stratospheric Sulfate Geoengineering on Global Solar Photovoltaic and Concentrating Solar Power Resource". Journal of Applied Meteorology and Climatology. 56 (5): 1483–1497. Bibcode:2017JApMC..56.1483S. doi:10.1175/JAMC-D-16-0298.1.
- HELIOSCSP. "Cement production with Concentrated Solar Power". helioscsp.com. Retrieved 20 October 2020.
- Izrael, Yuri; et al. (2009). "Field studies of a geoengineering method of maintaining a modern climate with aerosol particles". Russian Meteorology and Hydrology. 34 (10): 635–638. doi:10.3103/S106837390910001X. S2CID 129327083.
- Adler, Nils (2020-10-20). "10 million snowblowers? Last-ditch ideas to save the Arctic ice". The Guardian. ISSN 0261-3077. Archived from the original on 2020-10-27. Retrieved 2020-10-27.
- Dykema, John A.; et al. (2014). "Stratospheric controlled perturbation experiment: a small-scale experiment to improve understanding of the risks of solar geoengineering". Phil. Trans. R. Soc. A. 372 (2013): 20140059. Bibcode:2014RSPTA.37240059D. doi:10.1098/rsta.2014.0059. PMC 4240955. PMID 25404681.
- "SCoPEx Science". projects.iq.harvard.edu. Archived from the original on 2020-10-26. Retrieved 2020-10-27.
- Mason, Betsy (September 16, 2020). "Why solar geoengineering should be part of the climate crisis solution". Knowable Magazine. doi:10.1146/knowable-091620-2. Archived from the original on November 21, 2021. Retrieved June 29, 2021.
- Murdock, Jason (24 March 2021). "Bill Gates-funded Study to Dim Sunlight May Be Needed Against 'Horrific' Climate Change". Newsweek. Retrieved 13 March 2023.
- Cohen, ARiel. "A Bill Gates Venture Aims To Spray Dust Into The Atmosphere To Block The Sun. What Could Go Wrong?". Forbes. Retrieved 13 March 2023.
- Allan, Vicky (24 March 2021). "Bill Gates' chalk dust plan to save the world". The Herald. Retrieved 13 March 2023.
- "Research". Volcanic Emissions Group at the University of Bristol and Michigan Technological University. volcanicplumes.com. Archived from the original on 16 June 2021. Retrieved 3 April 2021.
- Hale, Erin (16 May 2012). "Controversial geoengineering field test cancelled". The Guardian. Archived from the original on 23 December 2013. Retrieved 25 May 2012.
- Pidgeon, Nick; Parkhill, Karen; Corner, Adam; Vaughan, Naomi (14 April 2013). "Deliberating stratospheric aerosols for climate geoengineering and the SPICE project" (PDF). Nature Climate Change. 3 (5): 451–457. Bibcode:2013NatCC...3..451P. doi:10.1038/nclimate1807. Archived (PDF) from the original on 19 January 2020. Retrieved 21 August 2021.
- Michael Marshall (3 October 2011). "Political backlash to geoengineering begins". New Scientist. Archived from the original on 21 March 2015. Retrieved 21 August 2021.
- "Open letter about SPICE geoengineering test". ETC Group. 27 Sep 2011. Archived from the original on 24 October 2011.
- Convention on Long-Range Transboundary Air Pollution art. 1, Nov. 13, 1979, 1302 U.N.T.S. 219, Article 1
- Vienna Convention for the Protection of the Ozone Layer, opened for signature Mar. 22, 1985, 1513 U.N.T.S. 293, Article 1
- Hester, Tracy D. (2011). "Remaking the World to Save It: Applying U.S. Environmental Laws to Climate Engineering Projects". Ecology Law Quarterly. 38 (4): 851–901. JSTOR 24115125. SSRN 1755203. Archived from the original on 2019-04-27. Retrieved 2020-07-11.
- "Patent US5003186 – Stratospheric Welsbach seeding for reduction of global warming – Google Patents". Google.com. Archived from the original on 2016-02-02. Retrieved 2016-01-10.
- Mario Sedlak: Physikalische Hindernisse bei der Umsetzung der im „Welsbach-Patent“ beschriebenen Idee In: Zeitschrift für Anomalistik. Bd. 15, 2015, ISSN 1617-4720, S. 317–325
- Rasch, P. J.; Tilmes, S.; Turco, R. P.; Robock, A.; Oman, L.; Chen, C.; Stenchikov, G. L.; Garcia, R. R. (Nov 2008). "An overview of geoengineering of climate using stratospheric sulphate aerosols". Philosophical Transactions of the Royal Society of London. Series A, Mathematical and Physical Sciences. 366 (1882): 4007–4037. Bibcode:2008RSPTA.366.4007R. doi:10.1098/rsta.2008.0131. ISSN 1364-503X. PMID 18757276. S2CID 9869660.
- "An overview of geoengineering of climate using stratospheric sulphate aerosols". Archived from the original on 2018-11-18. Retrieved 2021-11-16.
- "Nature's View of Geoengineering". 30 May 2012. Archived from the original on 2021-11-16. Retrieved 2021-11-16.
- Lapenis, A. (November 25, 2020). "A 50-Year-Old Global Warming Forecast That Still Holds Up". Eos Science News by AGU. Archived from the original on November 16, 2021. Retrieved November 16, 2021.
- Priday, Richard (August 8, 2018). "A volcano-inspired weapon to fix climate change is a terrible idea". Wired. Archived from the original on November 16, 2021. Retrieved November 16, 2021.
- Orquiola, John (4 February 2021). "Snowpiercer Theory: The World Is Warming Because Of The Train". SCREEN RANT. Retrieved 13 March 2023.
- Wehrstedt, Lisa (30 January 2021). "Snowpiercer season 2: Mr Wilford intentionally kicked off apocalypse - here's how". Express. Retrieved 13 March 2023.
- Robinson, Kim Stanley (2021). The ministry for the future (First paperback ed.). New York, NY: Orbit. ISBN 978-0-316-30013-1.
External links
- What can we do about climate change?, Oceanography magazine
- Global Warming and Ice Ages: Prospects for Physics-Based Modulation of Global Change, Lawrence Livermore National Laboratory
- The Geoengineering Option:A Last Resort Against Global Warming?, Council on Foreign Relations
- Geo-Engineering Climate Change with Sulfate Aerosols, Pacific Northwest National Laboratory
- Geo-Engineering Research, Parliamentary Office of Science and Technology
- Geo-engineering Options for Mitigating Climate Change, Department of Energy and Climate Change
- Unilateral Geoengineering, Council on Foreign Relations
- Rasch, Philip J; Tilmes, Simone; Turco, Richard P; Robock, Alan; Oman, Luke; Chen, Chih-Chieh (Jack); Stenchikov, Georgiy L; Garcia, Rolando R (13 November 2008). "An overview of geoengineering of climate using stratospheric sulphate aerosols". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 366 (1882): 4007–4037. Bibcode:2008RSPTA.366.4007R. doi:10.1098/rsta.2008.0131. PMID 18757276. S2CID 9869660.
- US 5003186 "Stratospheric Welsbach seeding for reduction of global warming"
- As planet warms, scientists explore 'far out' ways to reduce atmospheric CO2 on YouTube PBS NewsHour published on March 27, 2019 animation of SCoPEx