Strecker amino acid synthesis
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, is a method for the synthesis of amino acids by the reaction of an aldehyde with ammonia in the presence of potassium cyanide. The condensation reaction yields an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid.[1][2] The method is used commercially for the production of racemic methionine from methional.[3]

| Strecker synthesis | |
|---|---|
| Named after | Adolph Strecker |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | strecker-synthesis |
| RSC ontology ID | RXNO:0000207 |
While usage of ammonium salts gives unsubstituted amino acids, primary and secondary amines also give substituted amino acids. Likewise, the usage of ketones, instead of aldehydes, gives α,α-disubstituted amino acids.[4]
Reaction mechanism
In the first part of the reaction, the carbonyl oxygen of an aldehyde is protonated, followed by a nucleophilic attack of ammonia to the carbonyl carbon. After subsequent proton exchange, water is cleaved from the iminium ion intermediate. A cyanide ion then attacks the iminium carbon yielding an aminonitrile.
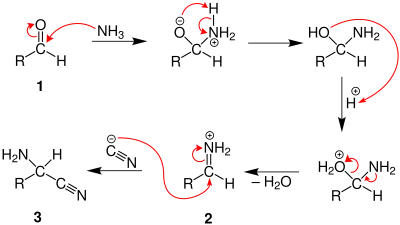
In the second part of the Strecker synthesis, the nitrile nitrogen of the aminonitrile is protonated, and the nitrile carbon is attacked by a water molecule. A 1,2-diamino-diol is then formed after proton exchange and a nucleophilic attack of water to the former nitrile carbon. Ammonia is subsequently eliminated after the protonation of the amino group, and finally the deprotonation of a hydroxyl group produces an amino acid.

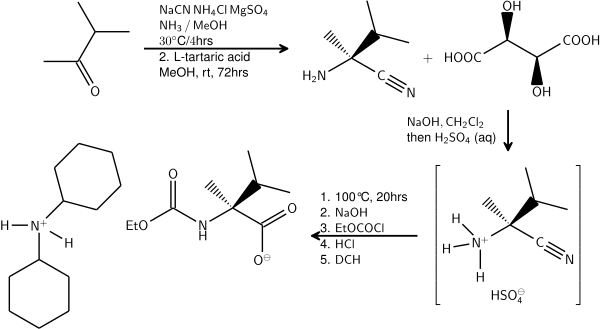
One example of the Strecker synthesis is a multikilogram scale synthesis of an L-valine derivative starting from Methyl isopropyl ketone:[5][6]
Asymmetric Strecker reactions
Asymmetric Strecker reactions are well developed. By replacing ammonia with (S)-alpha-phenylethylamine as chiral auxiliary the ultimate reaction product was chiral alanine.[7]
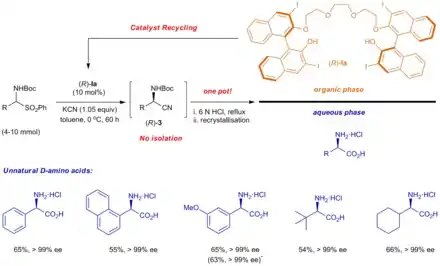
Catalytic asymmetric Strecker reaction can be effected using thiourea-derived catalysts.[8] In 2012, a BINOL-derived catalyst was employed to generate chiral cyanide anion.[9]
History
The German chemist Adolph Strecker discovered the series of chemical reactions that produce an amino acid from an aldehyde or ketone.[10][11] Using ammonia or ammonium salts in this reaction gives unsubstituted amino acids. In the original Strecker reaction acetaldehyde, ammonia, and hydrogen cyanide combined to form after hydrolysis alanine. Using primary and secondary amines in place of ammonium was shown to yield N-substituted amino acids.[11]
The classical Strecker synthesis gives racemic mixtures of α-amino acids as products, but several alternative procedures using asymmetric auxiliaries[12] or asymmetric catalysts[13][14] have been developed.
The asymmetric Strecker reaction was reported by Harada in 1963.[15] The first reported asymmetric synthesis via a chiral catalyst was published in 1996.[16] However, this was retracted in 2023.[17]
Commercial syntheses of amino acids
Several methods exist to synthesize amino acids aside from the Strecker synthesis.[18][3]
The commercial production of amino acids usually relies on mutant bacteria that overproduce individual amino acids using glucose as a carbon source. Otherwise amino acids are produced by enzymatic conversions of synthetic intermediates. 2-Aminothiazoline-4-carboxylic acid is an intermediate in one industrial synthesis of L-cysteine. Aspartic acid is produced by the addition of ammonia to fumarate using a lyase.[3]
One of the oldest methods begins with the bromination at the α-carbon of a carboxylic acid. Nucleophilic substitution with ammonia then converts the alkyl bromide to the amino acid.[19]
References
- "dl-ALANINE". Organic Syntheses. 9: 4. 1929. doi:10.15227/orgsyn.009.0004.
- "a-AMINOISOBUTYRIC ACID". Organic Syntheses. 11: 4. 1931. doi:10.15227/orgsyn.011.0004.
- Drauz, Karlheinz; Grayson, Ian; Kleemann, Axel; Krimmer, Hans-Peter; Leuchtenberger, Wolfgang; Weckbecker, Christoph (2006). "Amino Acids". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_057.pub2.
- Masumoto, Shuji; Usuda, Hiroyuki; Suzuki, Masato; Kanai, Motomu; Shibasaki, Masakatsu (May 2003). "Catalytic Enantioselective Strecker Reaction of Ketoimines". Journal of the American Chemical Society. 125 (19): 5634–5635. doi:10.1021/ja034980+. PMID 12733893.
- Kuethe, Jeffrey T.; Gauthier, Donald R.; Beutner, Gregory L.; Yasuda, Nobuyoshi (September 2007). "A Concise Synthesis of (S)-N-Ethoxycarbonyl-α-methylvaline". The Journal of Organic Chemistry. 72 (19): 7469–7472. doi:10.1021/jo7012862. PMID 17713956.
- The initial reaction product of 3-methyl-2butanone with sodium cyanide and ammonia is resolved by application of L-tartaric acid. The amino acid is isolated as its salt with dicyclohexylamine.
- Wang, Jun; Liu, Xiaohua; Feng, Xiaoming (9 November 2011). "Asymmetric Strecker Reactions". Chemical Reviews. 111 (11): 6947–6983. doi:10.1021/cr200057t. PMID 21851054.
- Zuend, Stephan J.; Coughlin, Matthew P.; Lalonde, Mathieu P.; Jacobsen, Eric N. (October 2009). "Scaleable catalytic asymmetric Strecker syntheses of unnatural α-amino acids". Nature. 461 (7266): 968–970. Bibcode:2009Natur.461..968Z. doi:10.1038/nature08484. PMC 2778849. PMID 19829379.
- Yan, Hailong; Suk Oh, Joong; Lee, Ji-Woong; Eui Song, Choong (20 November 2012). "Scalable organocatalytic asymmetric Strecker reactions catalysed by a chiral cyanide generator". Nature Communications. 3 (1): 1212. Bibcode:2012NatCo...3.1212Y. doi:10.1038/ncomms2216. PMID 23169053.
- Strecker, Adolph (1850). "Ueber die künstliche Bildung der Milchsäure und einen neuen, dem Glycocoll homologen Körper". Annalen der Chemie und Pharmacie. 75 (1): 27–45. doi:10.1002/jlac.18500750103.
- Strecker, Adolph (1854). "Ueber einen neuen aus Aldehyd - Ammoniak und Blausäure entstehenden Körper". Annalen der Chemie und Pharmacie. 91 (3): 349–351. doi:10.1002/jlac.18540910309.
- Davis, Franklin A.; Reddy, Rajarathnam E.; Portonovo, Padma S. (December 1994). "Asymmetric strecker synthesis using enantiopure sulfinimines: A convenient synthesis of α-amino acids". Tetrahedron Letters. 35 (50): 9351–9354. doi:10.1016/S0040-4039(00)78540-6.
- Ishitani, Haruro; Komiyama, Susumu; Hasegawa, Yoshiki; Kobayashi, Shū (February 2000). "Catalytic Asymmetric Strecker Synthesis. Preparation of Enantiomerically Pure α-Amino Acid Derivatives from Aldimines and Tributyltin Cyanide or Achiral Aldehydes, Amines, and Hydrogen Cyanide Using a Chiral Zirconium Catalyst". Journal of the American Chemical Society. 122 (5): 762–766. doi:10.1021/ja9935207.
- Huang, Jinkun; Corey, E. J. (December 2004). "A New Chiral Catalyst for the Enantioselective Strecker Synthesis of α-Amino Acids". Organic Letters. 6 (26): 5027–5029. doi:10.1021/ol047698w. PMID 15606127.
- Harada, Kaoru (December 1963). "Asymmetric Synthesis of α-Amino-acids by the Strecker Synthesis". Nature. 200 (4912): 1201. Bibcode:1963Natur.200.1201H. doi:10.1038/2001201a0. PMID 14089910. S2CID 43857409.
- Iyer, Mani S.; Gigstad, Kenneth M.; Namdev, Nivedita D.; Lipton, Mark (January 1996). "Asymmetric Catalysis of the Strecker Amino Acid Synthesis by a Cyclic Dipeptide". Journal of the American Chemical Society. 118 (20): 4910–4911. doi:10.1021/ja952686e. PMID 24178715.
- Iyer, Mani S.; Gigstad, Kenneth M.; Namdev, Nivedita D.; Lipton, Mark (2023-06-29). "Retraction of "Asymmetric Catalysis of the Strecker Amino Acid Synthesis by a Cyclic Dipeptide″". Journal of the American Chemical Society. doi:10.1021/jacs.3c03705. ISSN 0002-7863.
- Duthaler, Rudolf O. (January 1994). "Recent developments in the stereoselective synthesis of α-aminoacids". Tetrahedron. 50 (6): 1539–1650. doi:10.1016/S0040-4020(01)80840-1.
- McMurry J (1996). Organic chemistry. Pacific Grove, CA, USA: Brooks/Cole. p. 1064. ISBN 978-0-534-23832-2.