Succinaldehyde
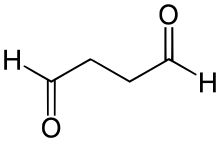
Succinaldehyde or succindialdehyde is an organic compound with the formula (CH2CHO)2. Typical of other dialdehydes, succinaldehyde is highly reactive and is rarely observed as the dialdehyde. Usually, it is handled as the hydrates or methanol-derived acetal. It is a precursor to tropinone.[2] Succinaldehyde can used as a crosslinking agent for proteins, but it is less widely used than the related dialdehyde glutaraldehyde.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Butanedial[1] | |
| Other names
Succinaldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.010.304 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H6O2 | |
| Molar mass | 86.09 |
| Appearance | colourless liquid |
| Density | 1.064 g/cm3 |
| Boiling point | 58 °C (136 °F; 331 K) at 9 mm Hg |
| with hydration | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation and reactions
Succinaldehyde is generated by the oxidation of tetrahydrofuran with chlorine followed by hydrolysis of the chlorinated product. It can also be prepared by the hydroformylation of acrolein or the acetals thereof.
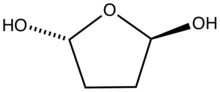
In aqueous solution, succinaldehyde quickly converts to the cyclic hydrate.[3] In methanol it converts to the cyclic acetal, 2,5-dimethoxyltetrahydrofuran.[4]
References
- International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 908. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- U.S. Patent 2,710,883
- Hardy, P. M.; Nicholls, A. C.; Rydon, H. N. (1972). "The Hydration and Polymerisation of Succinaldehyde, Glutaraldehyde, and Adipaldehyde". Journal of the Chemical Society, Perkin Transactions 2 (15): 2270. doi:10.1039/P29720002270.
- Christian Kohlpaintner; Markus Schulte; Jürgen Falbe; Peter Lappe; Jürgen Weber (2008). "Aldehydes, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_321.pub2.