Sulfenyl chloride
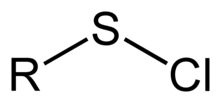
In organosulfur chemistry, a sulfenyl chloride is a functional group with the connectivity R−S−Cl, where R is alkyl[1] or aryl. Sulfenyl chlorides are reactive compounds that behave as sources of RS+. They are used in the formation of RS−N and RS−O bonds. According to IUPAC nomenclature they are named as alkyl thiohypochlorites, i.e. esters of thiohypochlorous acid.
Preparation
Sulfenyl chlorides are typically prepared by chlorination of disulfides:[2][3]
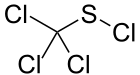
This reaction is sometimes called the Zincke disulfide reaction, in recognition of Theodor Zincke.[4][5] Typically, sulfenyl halides are stabilized by electronegative substituents. This trend is illustrated by the stability of CCl3SCl obtained by chlorination of carbon disulfide.
Some thioethers (R−S−R’) with electron-withdrawing substituents undergo chlorinolysis of a C−S bond to afford the sulfenyl chloride.[6][7]
Reactions
Perchloromethyl mercaptan (CCl3SCl) reacts with N−H bonds in the presence of base to give the sulfenamides:
This method is used in the production of the fungicides Captan and Folpet.
Sulfenyl chlorides add across alkenes, for example ethylene:[8]
They undergo chlorination to the trichlorides:[3]
Sulfenyl chlorides react with water and alcohols to give sulfenyl esters (R−SO−R′):[9]
Route to sulfinyl halides
Sulfenyl chlorides can be converted to sulfinyl chlorides (RS(O)Cl). In one approach, the sulfinyl chloride is generated in two steps starting with reaction of a thiol (−SH) with sulfuryl chloride (SO2Cl2). In some cases the sulfenyl chloride results instead, as happens with 2,2,2-trifluoro-1,1-diphenylethanethiol. A trifluoroperacetic acid oxidation then provides a general approach to formation of sulfinyl chlorides from sulfenyl chlorides:[10]
Cl.png.webp)
Related compounds
Sulfenyl fluorides and bromides are also known.[11] Simple sulfenyl iodides are unknown because they are unstable with respect to the disulfide and iodine:
Sulfenyl iodides can be isolated as stable compounds if they bear alkyl steric protecting groups as part of a cavity-shaped framework, illustrating the technique of kinetic stabilization of a reactive functionality, as in the case of sulfenic acids.[12]
A related class of compounds are the alkylsulfur trichlorides, as exemplified by methylsulfur trichloride, CH3SCl3.[13]
The corresponding selenenyl halides, R−SeCl, are more commonly encountered in the laboratory. Sulfenyl chlorides are used in the production of agents used in the vulcanization of rubber.
References
- Drabowicz, J.; Kiełbasiński, P.; Łyżwa, P.; Zając, A.; Mikołajczyk, M. (2008). Kambe, N. (ed.). Alkanesulfenyl Halides. Science of Synthesis. Vol. 39. pp. 544–550. ISBN 9781588905307.
- Hubacher, Max H. (1943). "o-Nitrophenylsulfur chloride". Organic Syntheses.; Collective Volume, vol. 2, p. 455
- Douglass, Irwin B.; Norton, Richard V. (1973). "Methanesulfinyl Chloride". Organic Syntheses.; Collective Volume, vol. 5, pp. 709–715
- Zincke, Th. (1911). "Über eine neue Reihe aromatischer Schwefelverbindungen". Chemische Berichte (in German). 44 (1): 769–771. doi:10.1002/cber.191104401109.
- Zincke, Th.; Farr, Fr. (1912). "Über o-Nitrophenylschwefelchlorid und Umwandlungsprodukte". Justus Liebig's Annalen der Chemie (in German). 391 (1): 57–88. doi:10.1002/jlac.19123910106.
- F. B. Wells, C. F. H. Allen (1935). "2,4-Dinitroaniline". Organic Syntheses. 15: 22. doi:10.15227/orgsyn.015.0022.
- Norman Kharasch, Robert B. Langford (1964). "2,4-Dinitrobenzenesulfenyl Chloride". Organic Syntheses. 44: 47. doi:10.15227/orgsyn.044.0047.
- Brintzinger, H.; Langheck, M., "Synthesen mit Alkylschwefelchloriden (X. Mitteil. über organische Schwefelchloride)", Chemische Berichte 1954, volume 87, 325-330. doi:10.1002/cber.19540870306
- Petrovic, Goran; Saicic, Radomir N.; Cekovic, Zivorad (2005). "Phenylsulfenylation of Nonactivated Carbon Atom by Photolysiis of Alkyl Benzenesulfenated: Preparation of 2-Phenylthio-5-heptanol". Organic Syntheses. 81: 244. doi:10.15227/orgsyn.081.0244.
- Page, P. C. B.; Wilkes, R. D.; Reynolds, D. (1995). "Alkyl Chalcogenides: Sulfur-based Functional Groups". In Ley, Steven V. (ed.). Synthesis: Carbon with One Heteroatom Attached by a Single Bond. Comprehensive Organic Functional Group Transformations. Elsevier. pp. 113–276. ISBN 9780080423234.
- Reno, Daniel S.; Pariza, Richard J. (1998). "Phenyl Vinyl Sulfide". Organic Syntheses.; Collective Volume, vol. 9, p. 662
- Sase, S.; Aoki, Y.; Abe, N.; Goto, K. (2009). "Stable Sulfenyl Iodide Bearing a Primary Alkyl Steric Protection Group with a Cavity-shaped Framework". Chemistry Letters. 38 (12): 1188–1189. doi:10.1246/cl.2009.1188.
- Braverman, S.; Cherkinsky, M.; Levinger, S. (2008). "Alkylsulfur Trihalides". Sci. Synth. 39: 187–188. ISBN 9781588905307.