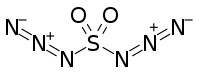
Sulfuryl diazide
Sulfuryl diazide or sulfuryl azide is a chemical compound with the molecular formula SO2(N3)2. It was first described in the 1920s when its reactions with benzene and p-xylene were studied by Theodor Curtius and Karl Friedrich Schmidt.[1][2][3] The compound is reported as having "exceedingly explosive, unpredictable properties" and "in many cases very violent explosions occurred without any apparent reason".[1]
 | |
| Names | |
|---|---|
| IUPAC name
Sulfuryl diazide | |
| Other names
Sulfuryl azide; Sulfonyl diazide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| SO2(N3)2 | |
| Molar mass | 148.10 g·mol−1 |
| Melting point | −15 °C (5 °F; 258 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It was not until 2011 that sulfuryl diazide was isolated in a pure enough state to be fully characterized.[4] It was characterized by infrared and Raman spectroscopy; its structure in the solid state was determined by x-ray crystallography.[4] Its melting point is -15 °C.[4] It was prepared by the reaction of sulfuryl chloride (SO2Cl2) with sodium azide (NaN3) using acetonitrile as solvent:
- SO2Cl2 + 2 NaN3 → SO2(N3)2 + 2 NaCl
Sulfuryl diazide has been used as a reagent to perform reactions that remove nitrogen from heterocyclic compounds:[5][6][7]
- R1−NH−R2 + SO2(N3)2 → R1−R2 + SO2 + 2 N2 + HN3
See also
References
- Curtius, Theodor; Schmidt, Friedrich (1922). "Action of sulfuryl azide, N3SO2N3, on p-xylene". Berichte der Deutschen Chemischen Gesellschaft B. 55B: 1571–1581.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Schmidt, Friedrich (1922). "Action of sulfuryl azide on benzene". Berichte der Deutschen Chemischen Gesellschaft B. 55B: 1581–1583. doi:10.1002/cber.19220550611.
- Schmidt, K. F. (1925). "Action of sulfuryl azide on benzene". Berichte der Deutschen Chemischen Gesellschaft B. 58B: 2409–2412. doi:10.1002/cber.19250581027.
- Xiaoqing Zeng, Helmut Beckers, Eduard Bernhardt, and Helge Willner (2011). "Synthesis and Characterization of Sulfuryl Diazide, O2S(N3)2". Inorg. Chem. 50 (17): 8679–8684. doi:10.1021/ic201294b. PMID 21815651.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Xiaodong Zou, Jiaqi Zou, Lizheng Yang, Guigen Li, and Hongjian Lu (2017). "Thermal Rearrangement of Sulfamoyl Azides: Reactivity and Mechanistic Study". J. Org. Chem. 82 (9): 4677–4688. doi:10.1021/acs.joc.7b00308. PMID 28414236.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Derek Lowe (July 7, 2021). "Carving Out Nitrogens: Pick Your Conditions". In The Pipeline. Science Translational Medicine. Archived from the original on June 5, 2023. Retrieved June 5, 2023.
- Qin, Haitao; Cai, Wangshui; Wang, Shuang; Guo, Ting; Li, Guigen; Lu, Hongjian (2021). "N‐Atom Deletion in Nitrogen Heterocycles". Angewandte Chemie International Edition. 60 (38): 20678–20683. doi:10.1002/anie.202107356. PMID 34227207. S2CID 235746021.