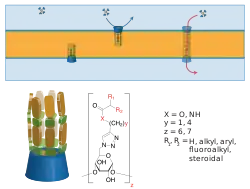
Synthetic ion channels
Synthetic ion channels are de novo chemical compounds that insert into lipid bilayers, form pores, and allow ions to flow from one side to the other.[1] They are man-made analogues of natural ion channels, and are thus also known as artificial ion channels. Compared to biological channels, they usually allow fluxes of similar magnitude but are
- minuscule in size (less than 5k Dalton vs. > 100k Dalton),
- diverse in molecular architecture, and
- may rely on diverse supramolecular interactions to pre-form the active, conducting structures.[2][3][4][5]
Synthetic channels, like natural channels, are usually characterized by a combination of single-molecule (e.g., voltage-clamp of planar bilayers[1]) and ensemble techniques (flux in vesicles[6]). The study of synthetic ion channels can potentially lead to new single-molecule sensing technologies as well as new therapeutics.
History

While semi-synthetic ion channels, often based on modified peptidic channels like gramicidin, had been prepared since the 1970s, the first attempt to prepare a synthetic ion channel was made in 1982 using a substituted β-cyclodextrin.[7]
Inspired by gramicidin, this molecule was designed to be a barrel-shaped entity spanning a single leaflet of a bilayer membrane, becoming "active" only when two molecules in opposite leaflets come together in an end-to-end fashion. While the compound does induce ion-fluxes in vesicles, the data does not unambiguously show channel formation (as opposed to other transport mechanisms; see Mechanism).
Na+ transport by such channels was first reported by two groups of investigators in 1989–1990.[8][9][10]
With the adoption of voltage clamp technique to synthetic channel research in the early 1990s, researchers were able to observe quantized electrical activities from synthetic molecules, often considered the signature evidence for ion channels.[1] This led to a sustained increase in research activity over the next two decades. In 2009, over 25 peer-reviewed papers were published on the topic,[11] and a series of comprehensive reviews are available.[3][4][5]
Characterization and mechanisms

Passive transport of ions across a membrane can take place by three main mechanisms: by ferrying, through defects in a disrupted membrane, or through a defined trajectory; these corresponds to ionophore, detergent, and ion channel transporters. While synthetic ion channel research attempts to prepare compounds that show conductance via a defined path, the elucidation of mechanism is difficult and seldom unambiguous. The two main methods of characterization both have their drawbacks, and as a consequence, often function is defined but mechanism presumed.
Ensemble, vesicle-based time course
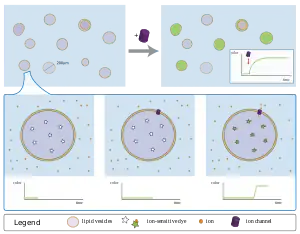
One line of evidence for ion transport comes from macroscopic examination of statistical ensembles. All these techniques use intact vesicles with an entrapped volume, with ion channel activities reported by different spectroscopic methods.[6]
In a typical case, a dye is entrapped within the population of vesicles. This dye is selected to be respond colorimetrically or fluorometrically to the presence of an ion; this ion is typically absent from the inside of the vesicle but present in the outside. Without an ion transporter, the lipid bilayer as a kinetic barrier to block ion flux, and the dye remains "dark" indefinitely.
As an ion transporter allows ions on the outside to diffuse in, its addition will affect the color/fluorescence property of the dye. By macroscopically monitoring the dye's properties over time, and controlling outside factors, the ability of a compound to act as an ion transporter can be measured.
Observing ion transport, however, does not pin down ion channel as the mechanism. Any class of transporter can lead to the same observation, and additional corroborating evidence is usually required. Sophisticated experiments intended to probe selectivity, gating, and other channel parameters have been developed over the past two decades and recently summarized.[6]
Vesicle assay variations
| Method | Reporter | Example |
|---|---|---|
| NMR | sodium-23 line width | Hydraphiles[12] |
| Fluorescent | HPTS ex/em ratios | Oligoesters[13] |
| pH-stat | H+ | Crown ether-barrels[14] |
| Ion-selective electrodes | Cl− | "SCMTR"[15] |
Stochastic, planar bilayer-based current traces

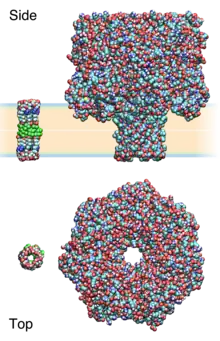
An alternative to the ensemble-based method described above is the voltage-clamp experiment.[16] In a voltage-clamp experiment, two compartments of electrolyte are divided by an aperture, usually between 5-250 micrometres in diameter. A lipid bilayer is painted across this aperture, thus electrically separating the compartments; the molecular nature can be ascertained by measuring its capacitance.
Upon the addition of an (ideal) ion channel, a defined path between the two compartments is formed. Through this pore, ions flow down the potential and electrochemical gradient rapidly (>106/second), the maximum flux limited by the geometry and dimensions of the pore. At some later instant the pore may close or collapse, whereupon the current returns to zero. This open-state current, originating and amplified from a single-molecule event, is typically on the order of pA to nA, with time-resolution of approx. millisecond. Ideal or close-to-ideal events is termed "square-tops" in the literature, and have been considered as signature for a channel-based mechanism.
It is notable that the events observed at this scale are truly stochastic - that is, they are the result of random molecular collision and conformation changes. As the membrane area is much larger than that of a pore, multiple copies may open and close independently of one another, giving rise to the staircase like appearance (Panel C in figure); these ideal events are often modelled as Markov processes.
By using the activity grid notation,[17] synthetic ion channels studied with the voltage-clamp method during the period 1982-2010 have been critically reviewed.[18] While the ideal traces are most frequently analyzed and reported in the literature, many records are decidedly non-ideal, with a subset was shown to be fractal.[19] Developing methods for analyzing these non-ideal traces and clarifying their relationship to transport mechanism is an area of contemporary research.
Examples
A diverse and large pool of synthetic molecules have been reported to act as ion transporters in lipid membranes. A selection is described here to demonstrate the breadth of feasible structures and attainable functions. Comprehensive reviews for the literature up to 2010 are available in a tripartite series.[3][4][5]
By chemical structure
Most (but not all; see minimalist channels) synthetic channels have chemical structures substantially larger than typical small molecules (molecular weights ~1-5kDa). This originates from the need to be amphiphilic, that is, have both sufficient hydrophobic portions to allow partitioning into lipid bilayer, as well as polar or charged "headgroups" to assert a defined orientation and geometry with respect to the membrane.
Crown ethers-based
.svg.png.webp)
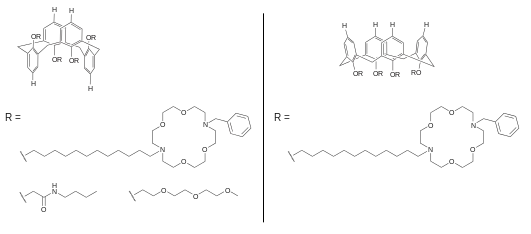
Calixarene-based
Ion channels containing calixarenes of ring size 3 and 4 have both been reported. For calix[4]arene, two conformations are accessible, and examples of both 1,3-alt and cone conformation have been developed.
Cyclodextrin-based
The first synthetic ion channel was constructed by partial substitution on the primary rim of β-cyclodextrin.[7] Other substituted β-cyclodextrins have since been reported, including thiol-modified cyclodextrins,[20] an anion-selective oligobutylene channel,[21] and various poly-ethyleneoxide linked starburst oligomers.[22] Structure-activity relationships for a large suite of cyclodextrin "half-channels" prepared by "click"-chemistry has been recently reported.[17]
Peptide-based
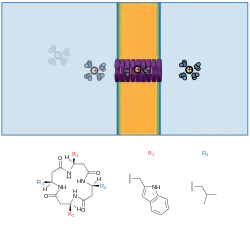
Alternating D/L peptide macrocycles are known to self-aggregate into nanotubes, and the resulting nanotubes have been shown to act as ion channels in lipid membranes.[23]
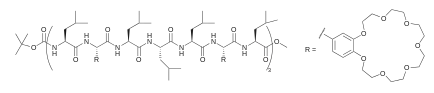
Other architectures use peptide helices as a scaffold to attach other functionalities, such as crown ethers of different sizes. The property of these peptide-crown channels depend strongly on the identity of the capping end-groups.
Minimalist channels
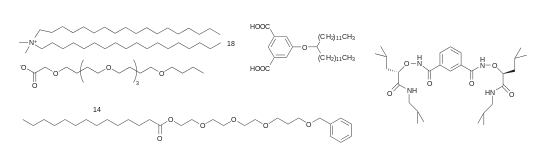
G-quartet based channels
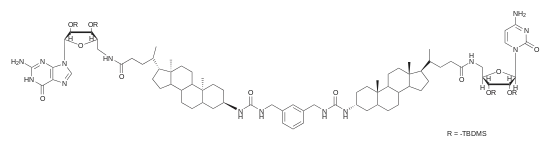
Hybrid bio-channels
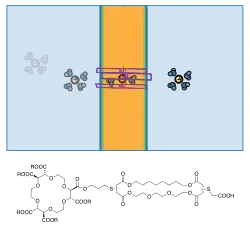
Semi-synthetic bio-hybrid channels constructed by modifications of natural ion channels had been constructed. Leveraging modern synthetic organic chemistry, these allows pinpoint modifications of existing structures to either elucidate their transport mechanisms or to graft on new functionalities.
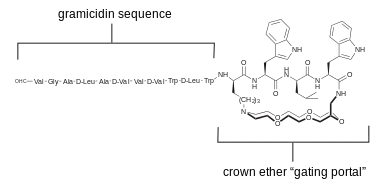
Gramicidin and alamethicin had been popular starting points for selective modifications.[24] The above diagram illustrates one example, where a crown-ether was fixed across the mouth of the ion-passing portal.[25] This channel shows discrete conductance but different ion selectivity than wild type gramicidin in voltage-clamp experiments.
While modification of large protein channels using mutagenesis are generally considered out of the scope of synthetic channels, the demarcation is not sharp, as supramolecular or covalent bonding of cyclodextrins to alpha-hemolysin demonstrates.[26]
By transport characteristics
An ion channel can be characterized by its opening characteristics, ion selectivity, and control of flux (gating). Many synthetic ion channels show unique properties in one or more of these aspects.
Opening characteristics
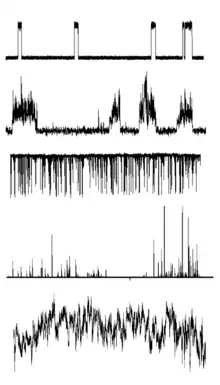
An "ion-channel forming" molecule can often show multiple types of conductance activities in planar bilayer membranes. Each of these modes of action can be characterized by their
- open duration (sub-ms---hours), related to whether the active structure is kinetically labile,
- unit conductance (pS---nS), related to the geometry of the active structure, and
- open probability, a fraction related to the thermodynamic stability of that active structure relative to inactive forms.
These events are not necessarily uniform throughout their durations, and as a result a variety of shapes of conducting traces are possible.
Ion selectivity
The majority of synthetic ion channels follow an Eisenman I sequence (Cs+ > Rb+ > K+ > Na+ >> Li+)[27] in their selectivity for alkali metal cations,[4][18] suggesting that the origin of the selectivity is governed by the difference in energy required to remove water from a fully hydrated cation. A few synthetic channels show other patterns of ion selectivity,[25] and only a single instance in which a synthetic channel following the opposite selectivity sequence (Eisenman XI; Cs+ < Rb+ < K+ < Na+ << Li+) had been reported.[28]
Voltage response

Most synthetic channels are Ohmic in conductance, that is, the current passed (both individually and as an ensemble) is proportional to the potential across the membrane. Some rare channels, however, show current-voltage characteristics that is non-linear. Specifically, two different types of non-Ohmic conductance are known:
- a rectifying behaviour, where current passes depends on the sign of the applied potential, and
- a exponential potential dependence, where the current passed scales exponentially with the applied potential.
The former requires asymmetry with respect to the mid-plane of the lipid bilayer, and is realized often by introducing an overall molecular dipole.[29][30][31] The latter, demonstrated in natural channels such as alamethicin, is rarely encountered in synthetic ion channels. They may be related to lipid ion channels, but to date their mechanism remains elusive.
Ligand response
Certain synthetic ion channels have conductances that can be modulated by additional of external chemicals. Both up-modulation (channels are turned on by ligand) and down-modulation (channels are turned off by ligands) are known: different mechanisms, including formation of supramolecular aggregates,[32][33] as well as inter- and intramolecular[34] blockage.
See also
References
- Fyles, TM (2007). "Synthetic ion channels in bilayer membranes". Chemical Society Reviews. 36 (2): 335–347. doi:10.1039/b603256g. PMID 17264934.
- Rodríguez-Vázquez, Nuria; Fuertes, Alberto; Amorín, Manuel; Granja, Juan R. (2016). "Chapter 14. Bioinspired Artificial Sodium and Potassium Ion Channels". In Astrid, Sigel; Helmut, Sigel; Roland K.O., Sigel (eds.). The Alkali Metal Ions: Their Role in Life. Metal Ions in Life Sciences. Vol. 16. Springer. pp. 485–556. doi:10.1007/978-3-319-21756-7_14. PMID 26860310.
- Matile, Stefan; Som, Abhigyan; Sorde, Nathalie (2004). "Recent Ion Channels and Pores". Tetrahedron. 60 (31): 6405–6435. doi:10.1016/j.tet.2004.05.052.
- Sisson, Adam L.; Shah, Muhammad Raza; Bhosale, Sheshanath; Matile, Stefan. (2006). "Synthetic Ion Channels and Pores (2004-2005)". Chemical Society Reviews. 35 (12): 1269–1286. doi:10.1039/b512423a. PMID 17225888.
- Matile, Stefan; Vargas Jentzsch, Andreas; Montenegro, Javier; Fin, Andrea (March 2011). "Recent Synthetic Transport Systems". Chemical Society Reviews. 40 (5): 2453–2474. doi:10.1039/C0CS00209G. PMID 21390363. S2CID 31753049.
- Matile, Stefan; Sakai, Naomi (2007). The characterization of synthetic ion channels and pores. Wiley VCH. doi:10.1002/9783527610273.ch11. ISBN 978-3-527-31505-5.
- Tabushi, Iwao; Kuroda, Yasuhisa; Yokota, Kanichi. (1982). "A,C,D,F-tetrasubstituted β-cyclodextrin as an artificial channel compound". Tetrahedron Letters. 23 (44): 4601–4604. doi:10.1016/S0040-4039(00)85664-6.
- V. E. Carmichael, P. J. Dutton, T. M. Fyles, T. D. James, J. A. Swan, M. Zojaji Biomimetic ion transport: a functional model of a unimolecular ion channel J. Am. Chem. Soc., 1989, 111, 767–769.
- A. Nakano, Q. Xie, J. V. Mallen, L. Echegoyen, G. W. Gokel Synthesis of a membrane-insertable, sodium cation conducting channel: kinetic analysis by dynamic sodium-23 NMR J. Am. Chem. Soc., 1990, 112, 1287–1289.
- G. W. Gokel, I. A. Carasel Biologically active, synthetic ion transporters Chem. Soc. Rev., 2007, 36, 378.
- Chui, JKW (2011). "Synthetic Ion Channel Bibliography". Archived from the original on June 21, 2011. Retrieved April 21, 2011.
- Ernesto Abel; Glenn E. M. Maguire; Eric S. Meadows; Oscar Murillo; Takashi Jin & George W. Gokel (September 1997). "Planar Bilayer Conductance and Fluorescence Studies Confirm the Function and Location of a Synthetic, Sodium-Ion-Conducting Channel in a Phospholipid Bilayer Membrane". Journal of the American Chemical Society. 119 (38): 9061–9062. doi:10.1021/ja971098t.
- Moszynski, J. M.; Fyles, T. M. (2010). "Synthesis, transport activity, membrane localization, and dynamics of oligoester ion channels containing diphenylacetylene units". Organic & Biomolecular Chemistry. 8 (22): 5139–5149. doi:10.1039/C0OB00194E. PMID 20835456. S2CID 22547440.
- Fyles, T. M.; James T. D.; Kaye K. T. (December 1993). "Activities and modes of action of artificial ion channel mimics". Journal of the American Chemical Society. 115 (26): 12315–12321. doi:10.1021/ja00079a011.
- Paul H. Schlesinger; Riccardo Ferdani; Jun Liu; Jolanta Pajewska; Robert Pajewski; Mitsuyoshi Saito; Hossein Shabany & George W. Gokel (2002). "SCMTR: A Chloride-Selective, Membrane-Anchored Peptide Channel that Exhibits Voltage Gating". Journal of the American Chemical Society. 124 (9): 1848–1849. doi:10.1021/ja016784d. PMID 11866586.
- Ashley, R. H. (1995). Ion Channels: A Practical Approach. Oxford: Oxford University Press. p. 302. ISBN 978-0-19-963474-3.
- Chui, J. K. W. (2011). A New Paradigm for the Voltage-Clamp Studies of Synthetic Ion Channels. Victoria BC, Canada: University of Victoria. p. 927.
- Chui, J. K. W.; Fyles, T. M. (June 2011). "Ionic conductance of synthetic channels: analysis, lessons, and recommendations". Chemical Society Reviews. 41 (1): 148–175. doi:10.1039/C1CS15099E. PMID 21691671.
- Chui, J. K. W.; Fyles, T. M. (May 2010). "Apparent fractal distribution of open durations in cyclodextrin-based ion channels". Chem. Comm. 46 (23): 4169–4171. doi:10.1039/C0CC00366B. PMID 20454723.
- Bacri, Laurent; Benkhaled, Amal; Guégan, Philippe; Auvray, Loïc (May 2005). "Ionic Channel Behavior of Modified Cyclodextrins Inserted in Lipid Membranes". Langmuir. 21 (13): 5842–5846. doi:10.1021/la047211s. PMID 15952831.
- Madhavan, Nandita; Robert, Erin C.; Gin, Mary S. (November 2005). "A Highly Active Anion-Selective Aminocyclodextrin Ion Channel". Angewandte Chemie International Edition. 44 (46): 7584–7587. doi:10.1002/anie.200501625. PMID 16247816.
- Badi, Nezha; Auvray, Loïc; Guégan, Philippe (October 2009). "Synthesis of Half-Channels by the Anionic Polymerization of Ethylene Oxide Initiated by Modified Cyclodextrin". Advanced Materials. 21 (40): 4054–4057. doi:10.1002/adma.200802982. S2CID 96554181.
- Ghadiri, M. Reza; Granja; Juan R.; Buehler, Lukas K. (May 1994). "Artificial transmembrane ion channels from self-assembling peptide nanotubes". Nature. 369 (6478): 301–304. Bibcode:1994Natur.369..301G. doi:10.1038/369301a0. PMID 7514275. S2CID 4241090.
- Shiroh Futaki; Masayuki Fukuda; Masayuki Omote; Kayoko Yamauchi; Takeshi Yagami; Mineo Niwa & Yukio Sugiura (2001). "Alamethicin−Leucine Zipper Hybrid Peptide: A Prototype for the Design of Artificial Receptors and Ion Channels". Journal of the American Chemical Society. 123 (49): 12127–12134. doi:10.1021/ja011166i. PMID 11734010.
- Jochen R. Pfeifer; Philipp Reiß; Ulrich Koert (2005). "Crown Ether–Gramicidin Hybrid Ion Channels: Dehydration-Assisted Ion Selectivity". Angewandte Chemie International Edition. 45 (3): 501–504. doi:10.1002/anie.200502570. PMID 16342124.
- Arijit Banerjee; Ellina Mikhailova; Stephen Cheley; Li-Qun Gu; Michelle Montoya; Yasuo Nagaoka; Eric Gouaux & Hagan Bayley (May 2010). "Molecular bases of cyclodextrin adapter interactions with engineered protein nanopores". PNAS. 107 (18): 8165–8170. Bibcode:2010PNAS..107.8165B. doi:10.1073/pnas.0914229107. PMC 2889592. PMID 20400691.
- Eisenman, George; Horn, Richard (1983). "Ionic selectivity revisited: The role of kinetic and equilibrium processes in ion permeation through channels". Journal of Membrane Biology. 76 (3): 197–225. doi:10.1007/BF01870364. PMID 6100862. S2CID 26390118.
- Jung, Minseon; Kim, Hyunuk; Baek, Kangkyun; Kim, Kimoon (June 2008). "Synthetic Ion Channel Based on Metal–Organic Polyhedra". Angewandte Chemie International Edition. 47 (31): 5755–5757. doi:10.1002/anie.200802240. PMID 18576447.
- Naomi Sakai; David Houdebert; Stefan Matile (December 2002). "Voltage-Dependent Formation of Anion Channels by Synthetic Rigid-Rod Push–Pull β-Barrels". Chemistry: A European Journal. 9 (1): 223–232. doi:10.1002/chem.200390016. PMID 12506379.
- Chigusa Goto; Mika Yamamura; Akiharu Satake & Yoshiaki Kobuke (2001). "Artificial Ion Channels Showing Rectified Current Behavior". Journal of the American Chemical Society. 123 (49): 12152–12159. doi:10.1021/ja010761h. PMID 11734013.
- T. M. Fyles; D. Loock & X. Zhou (1998). "A Voltage-Gated Ion Channel Based on a Bis-Macrocyclic Bolaamphiphile". Journal of the American Chemical Society. 120 (13): 2997–3003. doi:10.1021/ja972648q.
- Talukdar, Pinaki; Bollot, Guillaume; Mareda, Jiri; Sakai, Naomi; Matile, Stefan (August 2005). "Ligand-Gated Synthetic Ion Channels". Chemistry: A European Journal. 11 (22): 6525–6532. doi:10.1002/chem.200500516. PMID 16118825.
- Wilson, C. P.; Webb, S. J. (July 2008). "Palladium(II)-gated ion channels". Chem. Comm. (34): 4007–4009. doi:10.1039/B809087D. PMID 18758608. S2CID 27837694.
- G A Woolley; V Zunic; J Karanicolas; A S Jaikaran & A V Starostin (November 1997). "Voltage-dependent behavior of a "ball-and-chain" gramicidin channel". Biophysical Journal. 73 (5): 2465–2475. Bibcode:1997BpJ....73.2465W. doi:10.1016/S0006-3495(97)78275-4. PMC 1181148. PMID 9370440.
- Parag V. Jog & Mary S. Gin (2008). "A Light-Gated Synthetic Ion Channel". Organic Letters. 10 (17): 3693–3696. doi:10.1021/ol8013045. PMID 18656946.
- Vitali Borisenko; Darcy C. Burns; Zhihua Zhang & G. Andrew Woolley (June 2000). "Optical Switching of Ion−Dipole Interactions in a Gramicidin Channel Analogue". Journal of the American Chemical Society. 122 (27): 6343–6370. doi:10.1021/ja000736w.
- Matthew R. Banghart; Matthew Volgraf & Dirk Trauner (2006). "Engineering Light-Gated Ion Channels". Biochemistry. 45 (51): 15129–15141. CiteSeerX 10.1.1.70.6273. doi:10.1021/bi0618058. PMID 17176035.
- G. Andrew Woolley; Anna S. I. Jaikaran; Zhihua Zhang; Shuyun Peng (1995). "Design of Regulated Ion Channels Using Measurements of Cis-Trans Isomerization in Single Molecules". Journal of the American Chemical Society. 117 (16): 4448–4454. doi:10.1021/ja00121a002.