TMEM155
Transmembrane protein 155 is a protein that in humans is encoded by the TMEM155 gene. It is located on human chromosome 4, spanning 6,497 bases.[3] It is also referred to as FLJ30834 and LOC132332.[4] This protein is known to be expressed mainly in the brain, placenta, and lymph nodes and is conserved throughout most placental mammals.[5] The function and structure of this protein is still not well understood, but its level of expression has been studied pertaining to various pathologies.
| TMEM155 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | TMEM155, transmembrane protein 155 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | HomoloGene: 131149 GeneCards: TMEM155 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Gene
Locus
TMEM155 is located on the minus strand of human chromosome 4 (4q27) and spans 13,611 base pairs.[6]
Genetic Neighborhood
Cytogenetic band: 4q27[3]
TMEM155 is neighbored by TMEM155 is neighbored on chromosome 4 by CCNA2, a gene encoding for cyclin A2, and ANXA5, which encodes annexin A5.[4] It is also neighbored by PP12613 located on the positive strand.
Size
The gene on chromosome 4 encoding for TMEM155 spans 6,487 nucleotides.[5] This gene spans from base pairs 121,758,930 and 121,765,427 on chromosome 4.[3] The longest variant ofTMEM155 has 5 exons detailed in the table below:[5]
| Exon # | Base pairs | Length (bp) |
| 1 | 1-348 | 348 |
| 2 | 349-457 | 108 |
| 3 | 458-529 | 71 |
| 4 | 530-884 | 354 |
| 5 | 885-2429 | 1544 |
mRNA
Isoforms
There are 7 isoforms of TMEM155 precursor mRNA.[5] TMEM155 isoform 5 is the longest mRNA and is 2,429 bp long.[3] The shortest isoform is variant 4 and this variant is 2,035 bp long.[5] Isoforms are detailed in the table below.[5]
| Isoform Number | Length (bp) | Exons |
| Isoform 1 | 2,295 | 6 |
| Isoform 2 | 2,160 | 6 |
| Isoform 3 | 2,157 | 6 |
| Isoform 4 | 2,035 | 6 |
| Isoform 5 | 2,429 | 5 |
| Isoform 6 | 2,294 | 5 |
| Isoform 7 | 2,292 | 6 |
Protein
Primary Structure
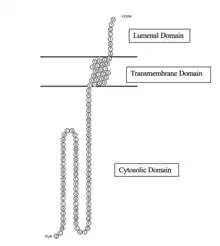
TMEM155 protein is 130 amino acids in length.[3] The TMEM155 protein in its full form is 14.2 kD in molecular weight with an isoelectric point of 10.29[7] Without its signal peptide it is 11.8 kD.[7] The protein interacts with the membrane once, with one transmembrane domain as seen below.
Secondary Structure
TMEM155 has a secondary structure composed of 23.5% alpha-helices, 67% beta-sheets, 9.5% turns and coils.[9]
Tertiary structure
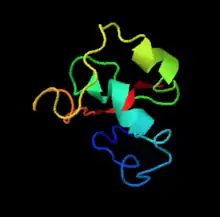
The tertiary structure of TMEM155 is predicted in the image on the right. This is predicted to be the structure of the N-terminus tail of TMEM155 located inside the ER membrane.
Post-translational modifications
TMEM155 has sites for O-glycosylation at ser78, thr79, and pro80.[11] It has sites for O-GlcNac at thr79 and ser121[12] It is a target for sumoylation from ile126 to val130.[13] There is a glycation site at lys102.[14]
Interacting Proteins
TMEM155 interacts with LMBR1 and TMEM259.[16] LMBR1 is a known lipocalin transmembrane receptor. TMEM259 is another transmembrane protein.
Regulation
Gene Level Regulation
There are several promoters of the TMEM155 gene.[17] The promoter region of the gene is bound by several transcription factors involved in regulating chromatin structure, development, cell cycle, and immune responses.[18] TMEM155 is expressed highly in the brain, placenta, and lymph nodes.[5] Below is a table detailing the transcription factor binding sites for the GXP_319937 promoter of TMEM155.[18] The table below details the transcription factors that bind the promoter region of TMEM155 and the sequences which they bind.
| Transcription factor | Detailed matrix information | Anchor base | Sequence |
| RUSH | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 3 | 29 | gtgtACTTttc |
| RUSH | 716 | tggaACTTtta | |
| BRAC | T-box transcription factor TBX20 | 96 | gtgctatgAGGTgtctgagtg |
| HOMF | Barx2, homeobox transcription factor that preferentially binds to paired TAAT motifs | 235 | aataaatTAATtgggaacg |
| HOMF | 232 | tcccaatTAATttatttcg | |
| FKHD | Alternative splicing variant of FOXP1, activated in ESCs | 303 | tttacaaAACAccagtc |
| FKHD | 16 | TTTACAAAACACCAGTC | |
| TF2B | Transcription factor II B (TFIIB) recognition element | 616 | ccgCGCC |
| RBP2 | Jumonji, AT rich interactive domain 1B | 1083 | GCACagcgc |
| EVI1 | MEL1 (MDS1/EVI1-like gene 1) DNA-binding domain 2 | 139 | cagtgaaGATGgggtct |
| SMAD | Smad3 transcription factor involved in TGF-beta signaling | 1071 | gggGTCTgggc |
| MYOD | Transcription factor E2a (E12/E47) | 605 | CAGCtg |
| ETSF | Ets variant 1 | 702 | gaagagcaGGAAgaagaa |
| ETSF | 366 | gtgcccgcGGAAgttcgctcc | |
| E2FF | E2F transcription factor 1 | 562 | gaggGGCGggagtgcgg |
| E2FF | 868 | cactGGCGggagggcac | |
| NFAT | Nuclear factor of activated T-cells | 467 | agctgaGGAAatccggcgc |
| NFAT | 488 | ctccgaGGAAacgcgccaa | |
| EGRF | Wilms Tumor Suppressor | 1018 | tcctgtgGGAGgcccgggg |
| STAT | Signal transducer and activator of transcription 3 | 944 | cagcTTCCaggtgcggggc |
Transcript Level Regulation
There are 4 splice enhancers of TMEM155.[17] These enhancer sites come on the 5' end of the TMEM155 gene and contain binding sites for transcription factors RCOR1, MILLT1, SIN3A, NFIC, STAT3, JUNB, FOS, EGR1, PHB2, RUNX3, and SRF.[17] Many of these transcription factors are involved with regulation cell growth and tumor suppression.
Mutations
There are several notable SNPs in the coding sequence of TMEM155. These mutations include mostly missense and nonsense mutations. The table below summarizes the mutations found in TMEM155 in the conserved bases.[19]
| Position in Protein | Mutation Type | Codon Position | Change in nucleic acid | Change in amino acid | Rs Number |
| 27 | Missense | 3 | G → A | M → I | rs754134166 |
| 28 | Missense | 1 | C → G | P → A | rs1056097623 |
| 34 | Nonsense | 1 | C → T | Q → STOP | rs148344547 |
| 44 | Missense | 2 | G → C | C → Y | rs1396459508 |
| 45 | Missense | 2 | A → G | H → R | rs761510691 |
| 49 | Missense | 3 | T → G | F → L | rs746407759 |
| 51 | Missense | 1 | G → A | G → R | rs1251128996 |
| 52 | Missense | 2 | T → C | M → T | rs1164776956 |
| 55 | Nonsense | 3 | G → A | C → STOP | rs749417444 |
| 56 | Missense | 1 | C → A | Q → K | rs1428301882 |
| 60 | Missense | 3 | G → C | L → F | rs756351338 |
| 61 | Missense | 1 | G → T | V → F | rs1268180828 |
| 65 | Missense | 1 | G → T | G → W | rs1344535938 |
| 65 | Missense | 2 | G → T | G → V | rs1267210743 |
| 68 | Missense | 1 | C → T | L → F | rs957334475 |
| 71 | Missense | 2 | G → A | R → K | rs1437581701 |
Evolution

TMEM155 is evolving at the molecular level rather quickly. When compared to fibrinogen protein rate of evolution, the TMEM155 appears to be accumulating more amino acid changes in a shorter amount of time. Because it is faster than the quickly evolving fibrinogen, it is also evolving faster than cytochrome C protein, which is known to evolve slowly.
Homology
TMEM155 is conserved across most placental mammals.[5] DoD (MYA) refers to how many million years ago the gene diverged from the human version of the gene.[20]
| Genus and Species | Common name | Taxomic group | DoD (MYA) | Accession number | Sequence length (aa) | E-value | Percent Identity | Percent Similarity |
| Homo sapiens | Human | Hominidae | 0 | NP_001304768.2 | 130 | 0.00E+00 | 100.00% | 100.00% |
| Pan troglodytes | Chimpanzee | Hominidae | 6.4 | XP_016807629.1 | 154 | 2.00E-87 | 99.00% | 99.00% |
| Pan paniscus | Bonobo | Hominidae | 6.4 | XP_008967732.1 | 130 | 7.00E-87 | 96.90% | 97.70% |
| Gorilla gorilla gorilla | Gorilla | Hominidae | 8.6 | XP_004040390.1 | 130 | 1.00E-88 | 99.20% | 99.20% |
| Pongo pygmaeus | Bornean orangutan | Hominidae | 15.2 | NP_001127639.1 | 130 | 2.00E-85 | 96.20% | 97.70% |
| Hylobates moloch | Silvery gibbon | Hylobatidae | 19.8 | XP_032002524.1 | 130 | 1.00E-84 | 95.40% | 96.90% |
| Propithecus coquereli | Coquerel's sifaka | Indriidae | 74.1 | XP_012505863.1 | 127 | 2.00E-68 | 79.80% | 84.60% |
| Fukomys damarensis | Damara mole-rat | Bathyeridae | 89 | XP_010609341.1 | 132 | 1.00E-52 | 69.70% | 77.30% |
| Oryctolagus cuniculus | European rabbit | Leporidae | 89 | XP_017203042.1 | 109 | 2.00E-39 | 52.90% | 58.80% |
| Camelus dromedarius | Dromedary | Camelidae | 94 | XP_031322500 | 106 | 7.00E-47 | 73.10% | 82.70% |
| Lynx canadensis | Canada Lynx | Felidae | 94 | XP_030169002 | 100 | 4.00E-44 | 70.20% | 76.90% |
| Bison bison bison | Bison | Bovidae | 94 | XP_010856646 | 190 | 3.00E-54 | 69.20% | 76.90% |
| Delphinapterus leucas | Beluga whale | Monodontidae | 94 | XP_022452038 | 100 | 6.00E-42 | 67.30% | 76.00% |
| Ceratotherium simum simum | Southern white rhinoceros | Rhinocerotidae | 94 | XP_014639974 | 192 | 4.00E-47 | 67.00% | 75.50% |
| Ursus arctos horribilis | Grizzly bear | Ursidae | 94 | XP_026355049.1 | 126 | 3.00E-52 | 66.20% | 72.20% |
| Neomonachus schauinslandi | Hawaiian monk seal | Phocidae | 94 | XP_021537176 | 126 | 9.00E-52 | 65.40% | 73.10% |
| Ailuropoda melanoleuca | Giant panda | Ursidae | 94 | XP_019660004 | 100 | 5.00E-40 | 63.60% | 70.10% |
| Mustela erminea | Stoat | Mustelidae | 94 | XP_032189210 | 127 | 8.00E-43 | 63.50% | 69.20% |
| Vicugna pacos | Alpaca | Camelidae | 94 | XP_015106166.1 | 106 | 3.00E-46 | 57.60% | 64.40% |
| Zalophus californianus | California sea lion | Otariidae | 94 | XP_027455522.1 | 109 | 4.00E-44 | 56.90% | 64.60% |
| Sus scrofa | Wild boar | Suidae | 94 | XP_020957297.1 | 104 | 7.00E-38 | 56.90% | 64.60% |
| Monodon monoceros | Narwhal | Monodontidae | 94 | XP_029091564.1 | 100 | 1.00E-42 | 53.80% | 61.50% |
| Panthera pardus | Leopard | Felidae | 94 | XP_019274438.1 | 98 | 1.00E-38 | 53.80% | 60.00% |
| Loxodonta africana | African bush elephant | Elephantidae | 102 | XP_023404270.1 | 127 | 2.00E-36 | 61.50% | 71.20% |
| Dasypus novemcinctus | Nine-banded armadillo | Dasypodidae | 102 | XP_023439327.1 | 103 | 1.00E-41 | 55.70% | 61.10% |
Clinical significance
Ocular tissues
The upregulation of TMEM155 has been found in basal cell nevus syndrome fibroblasts.[21] TMEM155 was also found to be upregulated in corneal keratinocytes,[22] which could contribute to the upregulation of the gene being associated with nystagmus.
Brain tissues
TMEM155 regulation co-varies with families that have instances of essential tremor,[23]
Female reproductive tissues
Hypermethylated TMEM155 is a potential biomarker for HER2+ breast cancer.[24] The expression of TMEM155 was found to be higher in the oocytes of women with low antral follicular count, meaning it could be involved in the regulation of female fertility.[25]
References
- GRCh38: Ensembl release 89: ENSG00000164112 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Gene: TMEM155 (ENSG00000164112) - Summary - Homo sapiens - Ensembl genome browser 99". useast.ensembl.org. Retrieved 2020-02-06.
- "AceView: Gene:TMEM155, a comprehensive annotation of human, mouse and worm genes with mRNAs or ESTsAceView". www.ncbi.nlm.nih.gov. Retrieved 2020-02-06.
- "TMEM155 transmembrane protein 155 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2020-02-06.
- "Home - Nucleotide - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2020-04-30.
- "SAPS < Sequence Statistics.< EMBL-EBI". www.ebi.ac.uk. Retrieved 2020-04-30.
- "SOSUI WWW Server". harrier.nagahama-i-bio.ac.jp. Retrieved 2020-04-30.
- "CFSSP: Chou & Fasman Secondary Structure Prediction Server". www.biogem.org. Retrieved 2020-04-30.
- "I-TASSER server for protein structure and function prediction". zhanglab.ccmb.med.umich.edu. Retrieved 2020-04-27.
- "5E94BCEC00000467232B8478 expired". www.cbs.dtu.dk. Retrieved 2020-04-30.
- "5E94B53300000468E744C097 expired". www.cbs.dtu.dk. Retrieved 2020-04-30.
- "GPS-SUMO: Prediction of SUMOylation Sites & SUMO-interaction Motifs". sumosp.biocuckoo.org. Retrieved 2020-04-30.
- "NetGlycate 1.0 Server". www.cbs.dtu.dk. Retrieved 2020-04-30.
- "PSORT WWW Server". psort.hgc.jp. Retrieved 2020-04-30.
- "Results - mentha: the interactome browser". mentha.uniroma2.it. Retrieved 2020-04-30.
- "UCSC Genome Browser Home". genome.ucsc.edu. Retrieved 2020-04-30.
- "Genomatix: Login Page". www.genomatix.de. Retrieved 2020-04-30.
- "SNP linked to Gene (geneID:132332) Via Contig Annotation". www.ncbi.nlm.nih.gov. Retrieved 2020-05-01.
- "TimeTree :: The Timescale of Life". www.timetree.org. Retrieved 2020-05-02.
- Renaud B, Buda M, Lewis BD, Pujol JF (September 1975). "Effects of 5,6-dihydroxytryptamine on tyrosine-hydroxylase activity in central catecholaminergic neurons of the rat". Biochemical Pharmacology. 24 (18): 1739–42. doi:10.1016/0006-2952(75)90018-0. PMID 17.
- Toyono T, Usui T, Yokoo S, Taketani Y, Nakagawa S, Kuroda M, et al. (2015-01-26). "Angiopoietin-like 7 is an anti-angiogenic protein required to prevent vascularization of the cornea". PLOS ONE. 10 (1): e0116838. Bibcode:2015PLoSO..1016838T. doi:10.1371/journal.pone.0116838. PMC 4306551. PMID 25622036.
- Odgerel Z, Sonti S, Hernandez N, Park J, Ottman R, Louis ED, Clark LN (2019). "Whole genome sequencing and rare variant analysis in essential tremor families". PLOS ONE. 14 (8): e0220512. Bibcode:2019PLoSO..1420512O. doi:10.1371/journal.pone.0220512. PMC 6690583. PMID 31404076.
- Lindqvist BM, Wingren S, Motlagh PB, Nilsson TK (August 2014). "Whole genome DNA methylation signature of HER2-positive breast cancer". Epigenetics. 9 (8): 1149–62. doi:10.4161/epi.29632. PMC 4164500. PMID 25089541.
- Barragán M, Pons J, Ferrer-Vaquer A, Cornet-Bartolomé D, Schweitzer A, Hubbard J, et al. (August 2017). "The transcriptome of human oocytes is related to age and ovarian reserve". Molecular Human Reproduction. 23 (8): 535–548. doi:10.1093/molehr/gax033. PMID 28586423.