Tetramethylxylylene diisocyanate
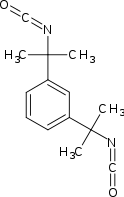
Tetramethylxylylene diisocyanate (TMXDI) is an organic compound with the formula C6H4(CMe2NCO)2 (Me = CH3). Introduced in the 1980s by American Cyanamid, the molecule features two isocyanate groups.[1][2][3] TMXDI is generally classified as an aliphatic isocyanate, which are generally more UV stable than their aromatic counterparts.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,3-Bis(2-isocyanatopropan-2-yl)benzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.614 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H16N2O2 | |
| Molar mass | 244.294 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.06 |
| Melting point | −10 °C (14 °F; 263 K) |
| Hazards | |
| GHS labelling: | |
    | |
| Danger | |
| H315, H317, H319, H330, H334, H335, H372, H410 | |
| P260, P261, P264, P270, P271, P272, P273, P280, P284, P285, P302+P352, P304+P340, P304+P341, P305+P351+P338, P310, P312, P314, P320, P321, P332+P313, P333+P313, P337+P313, P342+P311, P362, P363, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Production
Many isocyanates are produced by phosgenation of amines, but TMXDI is not. It is produced by the reaction of diisopropenylbenzene with hydrogen chloride followed by isocyanic acid:[4]
- C6H4(C(Me)=CH2)2 + 2 HCl → C6H4(CMe2Cl)2
- C6H4(CMe2Cl)2 + 2 HNCO → C6H4(CMe2NCO)2 + 2 HCl
Uses
A key use for TMXDI is in manufacturing polyurethane prepolymers. It is also used to manufacture polyurethane dispersions (PUDs).[5][6][7][8][9] These materials are then further used to manufacture coatings, adhesives, sealants and elastomers.
TMXDI has been considered as a replacement for Isophorone diisocyanate (IPDI). IPDI has a molecular weight of 222.3 and thus a NCO equivalent weight of 111.15. TMXDI has a molecular weight of 244.3 and thus an equivalent weight of 122.15. Thus per mole, approximately 10% more is required than the equivalent prepolymer based on IPDI. This difference increases cost.When making polyurethanes dispersions (PUDs) TMXDI is advantageous. Being sterically hindered, the NCO groups are slower reacting which is good when dispersing a prepolymer in water to make a PUD. It reduces side reactions and allows more time to allow the dispersion stage before the mix is chain extended. This is done usually with a diamine.[10] It has even found use in a rocket propellant binder by the US military.[11]
Safety
Extensive data has become available.[12]
See also
References
- "m-Tetramethylxylene diisocyanate". iaspub.epa.gov. United States Environmental Protection Agency. Archived from the original on 2018-11-06. Retrieved 2018-11-02.
- "1,3-Bis(1-isocyanato-1-methylethyl)benzene". pubchem.ncbi.nlm.nih.gov. National Center for Biotechnology Information, U.S. National Library of Medicine. Archived from the original on 2018-11-06. Retrieved 2018-10-23.
- "Tetramethylxylene diisocyanate". pubchem.ncbi.nlm.nih.gov. National Center for Biotechnology Information, U.S. National Library of Medicine. Archived from the original on 2018-11-06. Retrieved 2018-11-02.
- Christian Six; Frank Richter (2005). "Isocyanates, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a14_611.
- "Oxazolidines and tetramethylxylenediisocyanate based polyurethanes in legislation-compliant anticorrosion coatings – PDF Free Download". slideheaven.com. Retrieved 2018-10-23.
- US 7342068, Eric Howard Klingenberg & Shafiq Nisarali Fazel, "Aqueous polyurethane dispersion and method for making and using same", issued 2003-11-18, assigned to Versum Materials US LLC
- KG, Vincentz Network GmbH & Co. "Aqueous PUDs". European Coatings. Retrieved 2018-10-23.
- Howarth, G A; Manock, H L (July 1997). "Water-borne polyurethane dispersions and their use in functional coatings". Surface Coatings International. 80 (7): 324–328. doi:10.1007/bf02692680. ISSN 1356-0751. S2CID 137433262.
- "Water Based Polyurethanes Dispersions(PUDs)-An Overview". www.linkedin.com. Retrieved 2018-10-23.
- "TMXDI Technical Bulletin" (PDF).
- US 4913753, Marjorie E. Ducote, "TMXDI, curing agent for hydroxy terminated propellant binders", issued Apr. 3, 1990, assigned to The United States of America as represented by the Secretary of the Army, Washington, D.C.
- Pubchem. "1,3-Bis(1-isocyanato-1-methylethyl)benzene". pubchem.ncbi.nlm.nih.gov. Archived from the original on 2018-11-06. Retrieved 2018-10-23.