Tabernanthalog
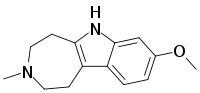
Tabernanthalog (TBG)[1] is a novel water-soluble, non-toxic azepinoindole[2] analog of the psychoactive drug ibogaine first synthesized by Professor David E. Olson at UC Davis.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
8-methoxy-3-methyl-2,4,5,6-tetrahydro-1H-azepino[4,5-b]indole | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C14H18N2O | |
| Molar mass | 230.311 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
In rodents, it was found to promote structural neural plasticity, reduce drug seeking behavior, and produce antidepressant like effects.[1][3][4][5] It has also been shown that it effectively reduces motivation for heroin and alcohol in rats. This indicates its efficacy in animals with a history of heroin and alcohol polydrug use.[6]
Due to the rapidly-induced and enduring neuroplasticity, Tabernanthalog is a member of the class of compounds known as non-hallucinogenic psychoplastogens.[1] This compound, as well as related compounds, are licensed by Delix Therapeutics and are being developed as potential medicines for neuropsychiatric disorders.[7]
References
- Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, et al. (January 2021). "A non-hallucinogenic psychedelic analogue with therapeutic potential". Nature. 589 (7842): 474–479. Bibcode:2021Natur.589..474C. doi:10.1038/s41586-020-3008-z. PMC 7874389. PMID 33299186.
- Hester JB, Tang AH, Keasling HH, Veldkamp W (January 1968). "Azepinoindoles. I. Hexahydroazepino[4,5-b]indoles". Journal of Medicinal Chemistry. 11 (1): 101–106. doi:10.1021/jm00307a023. PMID 5637151.
- Lu J, Tjia M, Mullen B, Cao B, Lukasiewicz K, Shah-Morales S, et al. (November 2021). "An analog of psychedelics restores functional neural circuits disrupted by unpredictable stress". Molecular Psychiatry. 26 (11): 6237–6252. doi:10.1038/s41380-021-01159-1. PMC 8613316. PMID 34035476.
- Peters J, Olson DE (2021-07-20). "Engineering Safer Psychedelics for Treating Addiction". Neuroscience Insights. 16: 26331055211033847. doi:10.1177/26331055211033847. PMC 8295933. PMID 34350400.
- Heinsbroek JA, Giannotti G, Bonilla J, Olson DE, Peters J (June 2023). "Tabernanthalog Reduces Motivation for Heroin and Alcohol in a Polydrug Use Model". Psychedelic Medicine. 1 (2): 111–119. doi:10.1089/psymed.2023.0009. PMC 10286262. PMID 37360328.
- Heinsbroek JA, Giannotti G, Bonilla J, Olson DE, Peters J (June 2023). "Tabernanthalog Reduces Motivation for Heroin and Alcohol in a Polydrug Use Model". Psychedelic Medicine. 1 (2): 111–119. doi:10.1089/psymed.2023.0009. PMC 10286262. PMID 37360328.
- Grace B (6 March 2021). "Can we take the high out of psychedelics?". Wired. Retrieved 12 July 2022.