Tartronic acid semialdehyde
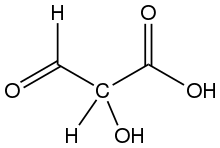
Tartronic acid semialdehyde is the organic compound with the formula OCHCH(OH)CO2H. The molecule has three functional groups, aldehyde, alcohol, and carboxylic acid. A white solid, it occurs naturally. A near neutral pH, it exists as the hydrated carboxylate (HO)2CHCH(OH)CO2−, which is referred to as tartronate semialdehyde. Tartronate semialdehyde is produced and consumed on a prodigious scale as an intermediate in photorespiration, an undesirable side reaction that competes with photosynthesis. It is produced biologically by the condensation of two equivalents of glyoxalate:[1]
- 2 OC(H)CO2H → OC(H)CH(OH)CO2H + CO2
 | |
| Names | |
|---|---|
| Other names
Tartronaldehydic acid 2-hydroxy-3-oxopropanoic acid formyl(hydroxy)acetic acid Hydroxymalonaldehydic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H4O4 | |
| Molar mass | 104.061 g·mol−1 |
| Appearance | white solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
This condensation is catalyzed by tartronate-semialdehyde synthase.
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.