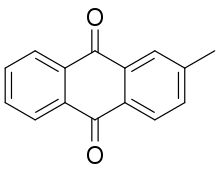
2-Methylanthraquinone
2-Methylanthraquinone, also known as β-methylanthraquinone and tectoquinone,[2] is an organic compound which is a methylated derivative of anthraquinone. An off-white solid, it is an important precursor to many dyes.[3] It is present in the wood of the teak tree, where it gives the tree resistance to insects.[4]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylanthracene-9,10-dione | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 2050523 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.399 |
| EC Number |
|
| 1607902 | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H10O2 | |
| Molar mass | 222.243 g·mol−1 |
| Appearance | almost colorless |
| Density | 1.365 g/cm3[1] |
| Melting point | 177 °C (351 °F; 450 K) |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H317, H410 | |
| P261, P272, P273, P280, P302+P352, P321, P333+P313, P363, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis and reactions
The compound is produced by the reaction of phthalic anhydride and toluene.[5][6] It can be chlorinated to give 1-chloro-2-methylanthraquinone. Nitration gives 1-nitro-2-methylanthraquinone, which can be reduced to 1-amino-2-methyl derivative. Oxidation of the methyl group gives anthraquinone-2-carboxylic acid.[3]
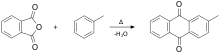
Summary eq for synthesis of 2-methylanthraquinone.
References
- Kingsford-Adaboh, R.; Kashino, S. (1995). "Disordered Structure of 2-Methylanthraquinone". Acta Crystallographica Section C. 51 (10): 2094–2096. doi:10.1107/S0108270195003842.
- "2-Methylanthraquinone". CAS Common Chemistry. Retrieved 5 August 2023.
- Bien, Hans-Samuel; Stawitz, Josef; Wunderlich, Klaus (2000). "Anthraquinone Dyes and Intermediates". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a02_355. ISBN 3527306730.
- Rudman, P.; Da Costa, E. W. B.; Gay, F. J.; Wetherly, A. H. (8 March 1958). "Relationship of tectoquinone to durability in Tectona grandis". Nature. 181 (4610): 721–722. doi:10.1038/181721b0.
- L. F. Fieser (1925). "p-Toluyl-o-Benzoic Acid". Organic Syntheses. 4: 73. doi:10.15227/orgsyn.004.0073.
- L. F. Fieser (1925). "β-Methylanthraquinone". Organic Syntheses. 4: 43. doi:10.15227/orgsyn.004.0043.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.