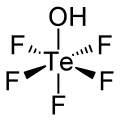
Teflic acid
Teflic acid is the chemical compound with the formula HOTeF5. This strong acid is related to orthotelluric acid, Te(OH)6. Teflic acid has a slightly distorted octahedral geometry.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Pentafluoroorthotelluric acid | |||
| Other names
Teflic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.161.534 | ||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| HF5OTe | |||
| Molar mass | 239.6 | ||
| Appearance | colorless solid | ||
| Melting point | 39.1 °C (102.4 °F; 312.2 K) | ||
| Boiling point | 59.7 °C (139.5 °F; 332.8 K) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
corrosive, toxic | ||
| GHS labelling: | |||
 | |||
| Danger | |||
| H314 | |||
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Preparation
Teflic acid was accidentally discovered by Engelbrecht and Sladky. Their synthesis did not yield the anticipated telluryl fluoride TeO2F2, but a mixture of volatile telluric compounds, containing HOTeF5:[1]
- BaTeO4 + 10HOSO2F → HOTeF5 (25%)
Teflic acid can also be prepared from fluorosulfonic acid and barium tellurate:[2]
- 5HOSO2F + BaO2Te(OH)4 → HOTeF5 + 4H2SO4 + BaSO4
It is also the first hydrolysis product of tellurium hexafluoride:
- TeF6 + H2O → HOTeF5 + HF
Teflates
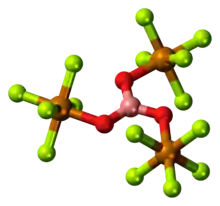
The conjugate base of teflic acid is called the teflate anion, F5TeO− (not to be confused with triflate). Many teflates are known, one example being B(OTeF5)3, that can be pyrolysed to give acid anhydride O(TeF5)2.[2]
- 2B(OTeF5)3 → 2 B(OTeF5)2F + O(TeF5)2
The teflate anion is known to resist oxidation. This property has allowed the preparation several highly unusual species such as the hexateflates M(OTeF5)−6 (in which M = As, Sb, Bi). Xenon forms the cation Xe(OTeF5)+.[3]
References
- Engelbrecht, A.; Sladky, F. "Pentafluoro-orthotellursaure, HOTeF5" Angewandte Chemie 1964. 76(9), 379-380, doi:10.1002/ange.19640760912.
- Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Mercier, H. P.A.; Sanders, J. C. P.; Schrobilgen, G. J. "The Hexakis(pentafluorooxotellurato)pnictate(V) Anions, M(OTeF5)−6 (M = As, Sb, Bi): A Series of Very Weakly Coordinating Anions" Journal of the American Chemical Society, volume 116, 2921, (1994). doi:10.1021/ja00086a025.
Further reading
- R.B. King; Inorganic Chemistry of Main Group Elements, VCH Publishers, New York,1994.