Tentoxin
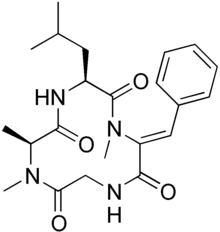
Tentoxin is a natural cyclic tetrapeptide produced by phytopathogenic fungus Alternaria alternata. It selectively induces chlorosis in several germinating seedling plants. Therefore, tentoxin may be used as a potential natural herbicide, and is a lactam.[1]
 | |
| Names | |
|---|---|
| IUPAC name
Cyclo(N-methyl-L-alanyl-L-leucyl-alpha,beta- | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C22H30N4O4 | |
| Molar mass | 414.498 g/mol |
| Melting point | 172 to 175 °C (342 to 347 °F; 445 to 448 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Tentoxin was first isolated from Alternaria alternata (syn. tenuis) and characterized by George Templeton et al. in 1967.[2]
Tentoxin has also been used in recent research to eliminate the polyphenol oxidase (PPO) activity from seedlings of higher plants.[3]
References
- PubChem. "Tentoxin". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-09-25.
- Templeton, G. E., C. 1. Grable, N. D. Fulton, W. L. Meyer. 1967. Tentoxin from Alternaria tenuis: its isolation and characterization. Proceedings of the Mycotoxin Research Seminar, Washington, D. C., June 8–9, 1967. United States Department of Agriculture. pp. 27-29
- Duke, S.O. & Vaughn, K.C. 1982. Lack of involvement of polyphenol oxidase in ortho-hydroxylation of phenolic compounds in mung bean seedlings. Physiol. Plant. 54: 381-385.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.