Axon terminal
Axon terminals (also called synaptic boutons, presynaptic terminals, or end-feet) are distal terminations of the branches of an axon. An axon, also called a nerve fiber, is a long, slender projection of a nerve cell that conducts electrical impulses called action potentials away from the neuron's cell body in order to transmit those impulses to other neurons, muscle cells or glands. In the central nervous system, most presynaptic terminals are actually formed along the axons (en-passant boutons), not at their ends (terminal boutons).
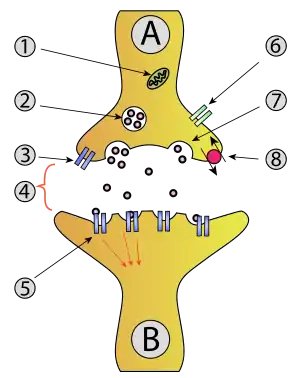
Functionally, the axon terminal converts an electrical signal into a chemical signal. When an action potential arrives at an axon terminal (A), neurotransmitter is released and diffuses across the synaptic cleft. If the postsynaptic cell (B) is also a neuron, neurotransmitter receptors generate a small electrical current that changes the postsynaptic potential. If the postsynaptic cell (B) is a muscle cell (neuromuscular junction), it contracts.
Neurotransmitter release
Axon terminals are specialized to release neurotransmitter very rapidly by exocytosis.[1] Neurotransmitter molecules are packaged into synaptic vesicles that cluster beneath the axon terminal membrane on the presynaptic side (A) of a synapse. Some of these vesicles are docked, i.e. connected to the membrane by a number of specialized proteins, the SNARE complex. The incoming action potential activates voltage-gated calcium channels, leading to an influx of calcium ions into the axon terminal. The SNARE complex reacts to these calcium ions and forces the membrane of the vesicle to fuse with the presynaptic membrane, releasing their content into the synaptic cleft within 180 µs of calcium entry.[2][3][4] When receptors in the postsynaptic membrane bind this neurotransmitter and open ion channels, information has been transmitted between neuron (A) and neuron (B).[5] To generate an action potential in the postsynaptic neuron, many excitatory synapses must be active at the same time.[1]
Imaging the activity of axon terminals
Historically, calcium-sensitive dyes were the first tool to quantify the calcium influx into synaptic terminals and to investigate the mechanisms of short-term plasticity.[6] The process of exocytosis can be visualized with pH-sensitive fluorescent proteins (Synapto-pHluorin): Before release, vesicles are acidic and the fluorescence is quenched. Upon release, they are neutralized, generating a brief flash of green fluorescence.[7] Another possibility is to construct a genetically encoded sensor that becomes fluorescent when bound to a specific neurotransmitter, e.g. glutamate.[8] This method is sensitive enough to detect the fusion of a single transmitter vesicle in brain tissue and to measure the release probability at individual synapses.[9]
See also
- Calyx of Held, a giant axon terminal in the auditory system
- Neuromuscular junction, axon terminal contacting a muscle cell
- Endocytosis to recycle vesicles after use
- Vesicular monoamine transporter, loading vesicles with neurotransmitter
- Optogenetic methods to measure cellular activity
References
- Purves, Dale; Augustine, George J.; Fitzpatrick, David, eds. (2019). Neuroscience (6th ed.). New York: Sinauer Associates / Oxford University Press. ISBN 978-1-60535-841-3.
- Llinás R, Steinberg IZ, Walton K (March 1981). "Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse". Biophysical Journal. 33 (3): 323–351. Bibcode:1981BpJ....33..323L. doi:10.1016/S0006-3495(81)84899-0. PMC 1327434. PMID 6261850.
- Rizo J (August 2018). "Mechanism of neurotransmitter release coming into focus". Protein Science (Review). 27 (8): 1364–1391. doi:10.1002/pro.3445. PMC 6153415. PMID 29893445.
Research for three decades and major recent advances have provided crucial insights into how neurotransmitters are released by Ca2+ -triggered synaptic vesicle exocytosis, leading to reconstitution of basic steps that underlie Ca2+ -dependent membrane fusion and yielding a model that assigns defined functions for central components of the release machinery.
- Südhof TC, Rizo J (December 2011). "Synaptic vesicle exocytosis". Cold Spring Harbor Perspectives in Biology. 3 (12): a005637. doi:10.1101/cshperspect.a005637. PMC 3225952. PMID 22026965.
- Siegelbaum, Steven A. (2021). Kandel, Eric R.; Koester, John D.; Mack, Sarah H. (eds.). Principles of neural science (6th ed.). New York: McGraw-Hill. ISBN 978-1-259-64223-4.
- Zucker RS, Regehr WG (2002). "Short-term synaptic plasticity". Annual Review of Physiology. 64 (1): 355–405. doi:10.1146/annurev.physiol.64.092501.114547. PMID 11826273.
- Burrone J, Li Z, Murthy VN (2006). "Studying vesicle cycling in presynaptic terminals using the genetically encoded probe synaptopHluorin". Nature Protocols. 1 (6): 2970–2978. doi:10.1038/nprot.2006.449. PMID 17406557.
- Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, Akerboom J, et al. (February 2013). "An optimized fluorescent probe for visualizing glutamate neurotransmission". Nature Methods. 10 (2): 162–170. doi:10.1038/nmeth.2333. PMC 4469972. PMID 23314171.
- Dürst CD, Wiegert JS, Schulze C, Helassa N, Török K, Oertner TG (October 2022). "Vesicular release probability sets the strength of individual Schaffer collateral synapses". Nature Communications. 13 (1): 6126. doi:10.1038/s41467-022-33565-6. PMC 9576736. PMID 36253353.
Further reading
- Cragg SJ, Greenfield SA (August 1997). "Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum". The Journal of Neuroscience. 17 (15): 5738–5746. doi:10.1523/JNEUROSCI.17-15-05738.1997. PMC 6573186. PMID 9221772.
- Vaquero CF, de la Villa P (October 1999). "Localisation of the GABA(C) receptors at the axon terminal of the rod bipolar cells of the mouse retina". Neuroscience Research. 35 (1): 1–7. doi:10.1016/S0168-0102(99)00050-4. PMID 10555158. S2CID 53189471.
- Roffler-Tarlov S, Beart PM, O'Gorman S, Sidman RL (May 1979). "Neurochemical and morphological consequences of axon terminal degeneration in cerebellar deep nuclei of mice with inherited Purkinje cell degeneration". Brain Research. 168 (1): 75–95. doi:10.1016/0006-8993(79)90129-X. PMID 455087. S2CID 19618884.
- Yagi T, Kaneko A (February 1988). "The axon terminal of goldfish retinal horizontal cells: a low membrane conductance measured in solitary preparations and its implication to the signal conduction from the soma". Journal of Neurophysiology. 59 (2): 482–494. doi:10.1152/jn.1988.59.2.482. PMID 3351572.
- LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite.Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D (November 1999). "LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite". Nature. 402 (6760): 421–425. Bibcode:1999Natur.402..421T. doi:10.1038/46574. PMID 10586883. S2CID 205056308.