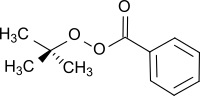
tert-Butyl peroxybenzoate
tert-Butyl peroxybenzoate (TBPB) an organic compound with the formula C6H5CO3CMe3 (Me = CH3). It is the most widely produced perester; it is an ester of peroxybenzoic acid (C6H5CO3H). It is often used as a radical initiator in polymerization reactions, such as the production of LDPE from ethylene, and for crosslinking, such as for unsaturated polyester resins.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
tert-Butyl benzenecarboperoxoate | |
| Other names
tert-Butyl perbenzoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.009.440 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H14O3 | |
| Molar mass | 194.230 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Properties
TBPB, which is pale yellow, is exclusively encountered as a solution in solvents such as ethanol or phthalate.[2]
As peroxo compound, TBPB contains about 8.16 wt% of active oxygen and has a self accelerating decomposition temperature (SADT) of about 60 °C. The SADT is the lowest temperature at which self-accelerating decomposition in the transport packaging can occur within a week, and which should not be exceeded while storage or transportation.[3] TBPB should therefore be stored between minimum 10 °C (below solidification) and maximum 50 °C. Dilution with a high-boiling solvent increases the SADT. The half-life of TBPB, in which 50% of the peroxy ester is decomposed, is 10 hours at 104 °C, one hour at 124 °C and one minute at 165 °C. Amines, metal ions, strong acids and bases, as well as strong reducing and oxidizing agents accelerate the decomposition of TBPB even in low concentrations.[3] However, TBPB is one of the safest peresters or organic peroxides in handling.[4] The main decomposition products of tert-butyl peroxybenzoate are carbon dioxide, acetone, methane, tert-butanol, benzoic acid and benzene.[5]
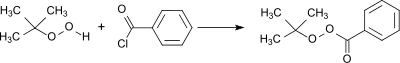
Production
A standard procedure for the preparation of peresters is the acylation of tert-butyl hydroperoxide with benzoyl chloride.[6] In the reaction a large excess of tert-butyl hydroperoxide is used and the hydrogen chloride formed is removed in vacuo whereby a virtually quantitative yield is obtained.
Applications
In polymer chemistry
Primarily, TBPB is used as a radical initiator, either in the polymerization of e.g. ethylene (to LDPE), vinyl chloride, styrene or acrylic esters or as so-called unsaturated polyester resins (UP resins).[2] The quantity used for the curing of UP resins is about 1-2%.[2]
A disadvantage, particularly in the production of polymers for applications in the food or cosmetics sector, is the possible formation of benzene as a decomposition product which can diffuse out of the polymer (for example, an LDPE packaging film).
In organic chemistry
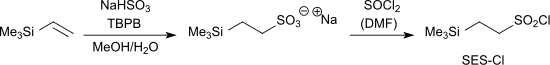
The protecting group 2-trimethylsilylethanesulfonyl chloride (SES-Cl) for primary and secondary amino groups is accessible by the reaction of vinyltrimethylsilane with sodium hydrogensulfite and TBPB to the sodium salt of trimethylsilylethanesulfonic acid and the subsequent reaction with thionyl chloride to the corresponding sulfonyl chloride.[4]
TBPB can be used to introduce a benzoyloxy group in the allyl position of unsaturated hydrocarbons.[7]
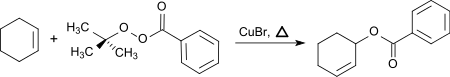
From cyclohexene, 3-benzoyloxycyclohexene is formed with TBPB in the presence of catalytic amounts of copper(I)bromide in 71 to 80% yield.
This allylic oxidation of alkenes, also known as Kharasch-Sosnovsky oxidation, generates racemic allylic benzoates in the presence of catalytic amounts of copper(I)bromide.[8]
A modification of the reaction utilizes copper(II) trifluoromethanesulfonate as a catalyst and DBN or DBU as bases to achieve yields up to 80% in the reaction of acyclic olefins with TBPB to allylic benzoates.[9]
Substituted oxazolines and thiazolines can be oxidized to the corresponding oxazoles and thiazoles in a modified Kharash-Sosnovsky oxidation with TBPB and a mixture of Cu(I) and Cu(II) salts in suitable yields.[10]
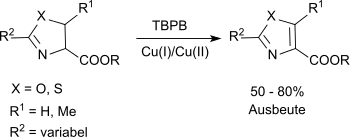
The carboalkoxy group at the C-4 position is essential a successful reaction.
Benzene and furans can be alkenylated with olefins in an oxidative coupling under palladium salt catalysis, with TBPB as hydrogen acceptor.[11]
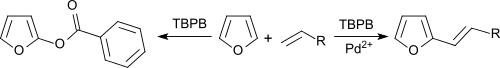
In the absence of Pd2+ salts, the aromatics are benzoxylated.
References
- Klenk, Herbert; Götz, Peter H.; Siegmeier, Rainer; Mayr, Wilfried. "Peroxy Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_199.pub2.
- United Initiators, Technisches Datenblatt, TBPB
- Organic Peroxide Producers Safety Division, SAFETY AND HANDLING OF ORGANIC PEROXIDES The Society of the Plastics Industry, 2012 edition Archived 2016-04-01 at the Wayback Machine
- "2-Trimethylsilylethanesulfonyl chloride (SES-Cl)". Organic Syntheses. doi:10.15227/orgsyn.075.0161.
- PERGAN GmbH: Organische Peroxide für die Polymerisation
- N.A. Milas, D.G. Orphanos, R.J. Klein (1964), "The solvolysis of acid chlorides with t-alkyl hydroperoxides", J. Org. Chem., vol. 29, no. 10, pp. 3099–3100, doi:10.1021/jo01033a525
{{citation}}: CS1 maint: multiple names: authors list (link) - "3-Benzoyloxycyclohexene". Organic Syntheses. doi:10.15227/orgsyn.048.0018.
- M.S. Kharasch, G. Sosnovsky (1958), "The reactions of t-butyl perbenzoate and olefins – a stereospecific reaction", J. Am. Chem. Soc., vol. 80, no. 3, p. 756, doi:10.1021/ja01536a062
- G. Sakar, A. DattaGupta, V.K. Singh (1996), "Cu(OTf)2 – DBN/DBU complex as an efficient catalyst for allylic oxidation of olefins with tert-butyl perbenzoate", Tetrahedron Lett., vol. 37, no. 46, pp. 8435–8436, doi:10.1016/0040-4039(96)01911-9
{{citation}}: CS1 maint: multiple names: authors list (link) - A.I. Meyers, F.X. Tavares (1996), "Oxidation of Oxazolines and Thiazolines to Oxazoles and Thiazoles. Application of the Kharasch−Sosnovsky Reaction", J. Org. Chem., vol. 61, no. 23, pp. 8207–8215, doi:10.1021/jo9613491, PMID 11667808
- J. Tsuji, H. Nagashima (1984), "Palladium-catalyzed oxidative coupling of aromatic compounds with olefins using t-butyl perbenzoate as a hydrogen accepter", Tetrahedron, vol. 40, no. 14, pp. 2699–2702, doi:10.1016/S0040-4020(01)96888-7