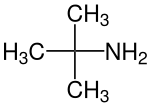
tert-Butylamine
tert-Butylamine is an organic chemical compound with the formula (CH3)3CNH2. It is a colorless liquid with a typical amine-like odor. tert-Butylamine is one of the four isomeric amines of butane, the others being n-butylamine, sec-butylamine and isobutylamine.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methylpropan-2-amine | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| 605267 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.808 | ||
| EC Number |
| ||
| 1867 | |||
| MeSH | tert-butylamine | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3286 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H11N | |||
| Molar mass | 73.139 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | fishy, ammoniacal | ||
| Density | 0.696 g/mL | ||
| Melting point | −67.50 °C; −89.50 °F; 205.65 K | ||
| Boiling point | 43 to 47 °C; 109 to 116 °F; 316 to 320 K | ||
| Miscible | |||
| log P | 0.802 | ||
| Vapor pressure | 39.29 kPa (at 20 °C) | ||
Refractive index (nD) |
1.377 | ||
| Thermochemistry | |||
Heat capacity (C) |
191.71 J K−1 mol−1 | ||
Std molar entropy (S⦵298) |
233.63 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
−151.1–−150.1 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) |
−2.9959–−2.9951 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
   | |||
| Danger | |||
| H225, H302, H314, H331 | |||
| P210, P261, P280, P305+P351+P338, P310 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −38 °C (−36 °F; 235 K) | ||
| 380 °C (716 °F; 653 K) | |||
| Explosive limits | 1.7–9.8% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
464 mg kg−1 (oral, rat) | ||
| Safety data sheet (SDS) | rose-hulman.edu | ||
| Related compounds | |||
Related alkanamines |
|||
Related compounds |
2-Methyl-2-nitrosopropane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Preparation
tert-Butylamine is produced commercially by direct amination of isobutylene using zeolite catalysts:
- NH3 + CH2=C(CH3)2 → H2NC(CH3)3
The Ritter reaction of isobutene with hydrogen cyanide is not useful because it produces too much waste.[1]
- (CH3)2C=CH2 + HCN + H2O → (CH3)3CNHCHO
- (CH3)3CNHCHO + H2O → (CH3)3CNH2 + HCO2H
In the laboratory, it can be prepared by the hydrogenolysis of 2,2-dimethylethylenimine, or via tert-butylphthalimide.[2]
Uses
tert-Butylamine is used as an intermediate in the preparation of the sulfenamides such as N-tert-butyl-2-benzothiazylsulfenamide and N-tert-butyl-2-benzothiazylsulfenimide. As rubber accelerators, these compounds modify the rate of vulcanization of rubber. A variety of pesticides are derived from this amine, including terbacil, terbutryn, and terbumeton.
In pharmacology under the name erbumine, tert-butylamine has been used as a counterion in drug substances such as perindopril erbumine.
See also
References
- Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2000). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001.
- Kenneth N. Campbell, Armiger H. Sommers, Barbara K. Campbell (1947). "tert'-Butylamine". Organic Syntheses. 47: 12. doi:10.15227/orgsyn.027.0012.
{{cite journal}}: CS1 maint: multiple names: authors list (link)