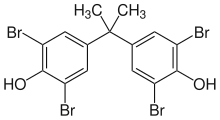
Tetrabromobisphenol A
Tetrabromobisphenol A (TBBPA) is a brominated flame retardant. The compound is a white solid (not colorless), although commercial samples appear yellow. It is one of the most common flame retardants.[2]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4,4′-(Propane-2,2-diyl)bis(2,6-dibromophenol) | |
| Other names
2,2′,6,6′-Tetrabromobisphenol A, 2,2′,6,6′-Tetrabromo-4,4′-isopropylidenediphenol, 2,2-Bis(3,5-dibromo-4-hydroxyphenyl)propane, 4,4′-Isopropylidenebis(2,6-dibromophenol) | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | TBBPA, TBBP-A, TBBA |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.125 |
| EC Number |
|
| KEGG | |
| MeSH | C443737 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H12Br4O2 | |
| Molar mass | 543.9 g·mol−1 |
| Density | 2,12 g·cm−3 (20 °C)[1] |
| Melting point | 178 °C (352 °F; 451 K)[1] |
| Boiling point | 250 °C (482 °F; 523 K) (decomposition)[1] |
| insoluble | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
N[1] |
| GHS labelling: | |
 | |
| Warning | |
| H410 | |
| P273, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Production and use
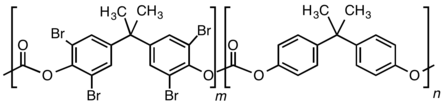
TBBPA is produced by the reaction of bromine with bisphenol A. Most commercial TBBPA products consist of a mixture that differ in the degree of bromination with the formula C15H16−xBrxO2 where x = 1 to 4. Its fire-retarding properties correlate with its bromine content. The annual consumption in Europe has been estimated as 6200 tons in 2004.[3]
TBBPA is mainly used as a reactive component of polymers, meaning that it is incorporated into the polymer backbone. It is used to prepare fire-resistant polycarbonates by replacing some bisphenol A. A lower grade of TBBPA is used to prepare epoxy resins, used in printed circuit boards.[2]
Toxicity
A study was published by the European Food Safety Authority (EFSA) in December 2011 on the exposure of TBBPA and its derivatives in food. The study, which examined at 344 food samples from the fish and other seafood food group, concluded that “current dietary exposure to TBBPA in the European Union does not raise a health concern.” EFSA also determined that “additional exposure, particularly of young children, to TBBPA from house dust is unlikely to raise a health concern”.[4]
Some studies suggest that TBBPA may be an endocrine disruptor and immunotoxicant. As an endocrine disruptor, TBBPA may interfere with both estrogens and androgens.[5] Further, TBBPA structurally mimics the thyroid hormone thyroxin (T4) and can bind more strongly to the transport protein transthyretin than T4 does, likely interfering with normal T4 activity. TBBPA likely also suppresses immune responses by inhibiting expression of CD25 receptors on T cells, preventing their activation, and by reducing natural killer cell activity.[6][7]
A 2013 literature review on TBBPA concludes that TBBPA does not produce “adverse effects that might be considered to be related to disturbances in the endocrine system”.[8] Therefore, in accordance with internationally accepted definitions, TBBPA should not be considered an “endocrine disruptor”. Furthermore, TBBPA is rapidly excreted in mammals and therefore does not have a potential for bioaccumulation. Measured concentrations of TBBPA in house dust, human diet and human serum samples are very low. Daily intakes of TBBPA in humans were estimated to not exceed a few ng/kg bw/day. Exposures of the general population are also well below the derived-no-effect-levels (DNELs) derived for endpoints of potential concern in REACH.
TBBPA degrades to bisphenol A and to TBBPA dimethyl ether, and experiments in zebrafish (Danio rerio) suggest that during development, TBBPA may be more toxic than either BPA or TBBPA dimethyl ether.[9]
Occurrence
TBBPA emits can be found in trace concentration in the hydrosphere, soil, and sediments.[10] It also occurs in sewage sludge and house dust.[11] TBBPA has been the subject of an eight-year evaluation under the EU Risk Assessment procedure which reviewed over 460 studies. The Risk Assessment was published on the EU Official Journal in June 2008.[12] The conclusions of the Risk Assessment were confirmed by the European Commission SCHER Committee (Scientific Committee on Health and Environmental Risks[13]). TBBPA has been registered under REACH.[14]
See also
Further reading
- Early work on bromination of BPA: Zincke, T. (1905). "Ueber die Einwirkung von Brom und von Chlor auf Phenole: Substitutionsprodukte, Pseudobromide und Pseudochloride". Justus Liebigs Annalen der Chemie. 343: 75–99. doi:10.1002/jlac.19053430106.
References
- Record of Tetrabromobisphenol A in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2008/2/15.
- Dagani, M. J.; Barda, H. J.; Benya, T. J.; Sanders, D. C. "Bromine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_405.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - "RISK ASSESSMENT OF 2,2',6,6'-TETRABROMO-4,4'-ISOPROPYLIDENE DIPHENOL (TETRABROMOBISPHENOL-A)". Environment Agency (UK). June 2007.
- EFSA Scientific Opinion on Tetrabromobisphenol A (TBBPA) and its derivatives in food (2011) https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2011.2477. See page 1.
- Shaw, S.; Blum, A.; Weber, R.; Kannan, K.; Rich, D.; Lucas, D.; Koshland, C.; Dobraca, D.; Hanson, S.; Birnbaum, L. (2010). "Halogenated flame retardants: do the fire safety benefits justify the risks?". Reviews on Environmental Health. 25 (4): 261–305. doi:10.1515/REVEH.2010.25.4.261. PMID 21268442. S2CID 20573319.
- Pullen, S; Boecker R.; Tiegs G (2003). "The flame retardants tetrabromobisphenol A and tetrabromobisphenol A–bisallylether suppress the induction of interleukin-2 receptor α chain (CD25) in murine splenocytes". Toxicology. 184 (1): 11–22. doi:10.1016/S0300-483X(02)00442-0. PMID 12505372.
- Kibakaya, EC; Stephen K; Whalen MM (2009). "Tetrabromobisphenol A has immunosuppressive effects on human natural killer cells". Journal of Immunotoxicology. 6 (4): 285–292. doi:10.3109/15476910903258260. PMC 2782892. PMID 19908946.
- Colnot, Thomas; Kacew, Sam; Dekant, Wolfgang (2013). "Mammalian toxicology and human exposures to the flame retardant 2,2′,6,6′-tetrabromo-4,4′-isopropylidenediphenol (TBBPA): implications for risk assessment". Archives of Toxicology. 88 (3): 553–73. doi:10.1007/s00204-013-1180-8. PMID 24352537. S2CID 15254375.
- McCormick, J; Paiva MS; Häggblom MM; Cooper KR; White LA (2010). "Embryonic exposure to tetrabromobisphenol A and its metabolites, bisphenol A and tetrabromobisphenol A dimethyl ether disrupts normal zebrafish (Danio rerio) development and matrix metalloproteinase expression". Aquatic Toxicology. 100 (3): 255–262. doi:10.1016/j.aquatox.2010.07.019. PMC 5839324. PMID 20728951.
- Covaci, Adrian; Voorspoels, Stefan; Abdallah, Mohamed Abou-Elwafa; Geens, Tinne; Harrad, Stuart; Law, Robin J. (January 2009). "Analytical and environmental aspects of the flame retardant tetrabromobisphenol-A and its derivatives". Journal of Chromatography A. 1216 (3): 346–363. doi:10.1016/j.chroma.2008.08.035. PMID 18760795.
- Kuch B, Körner W, Hagenmaier H (2001): Monitoring von bromierten Flammschutzmitteln in Fliessgewässern, Abwässern und Klärschlämmen in Baden-Württemberg Archived 2003-12-29 at the Wayback Machine. Umwelt und Gesundheit, Universität Tübingen.
- TBBPA draft RAR
- European Union Risk Assessment Report on TBBPA (2008) http://echa.europa.eu/documents/10162/32b000fe-b4fe-4828-b3d3-93c24c1cdd51
- TBBPA REACH Registration webpage http://echa.europa.eu/web/guest/information-on-chemicals/registered-substances
External links
- BSEF, TBBPA Factsheet
- BSEF, industry page on TBBPA: Environmental aspects