Tetrabutylammonium chloride
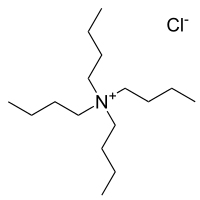
Tetrabutylammonium chloride is the organic compound with the formula (C4H9)4NCl. A white water-soluble solid, it is a quaternary ammonium salt of chloride. It is a precursor to other tetrabutylammonium salts.[1][2] Often tetrabutylammonium bromide is preferred as a source of tetrabutylammonium because it is less hygroscopic than the chloride.[3]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| 3571227 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.012.905 |
| EC Number |
|
| 10839 | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H36ClN | |
| Molar mass | 277.92 g·mol−1 |
| Appearance | white solid |
| Density | 1.018 g/cm3 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Barder, T. J.; Walton, R. A. (1985). "Tetrabutylammonium Octachlorodirhenate(III)". Inorganic Syntheses. pp. 116–118. doi:10.1002/9780470132548.ch22. ISBN 9780470132548.
{{cite book}}:|journal=ignored (help) - Dilworth, J. R.; Hussain, W.; Hutson, A. J.; Jones, C. J.; McQuillan, F. S. (1997). "Tetrahalo Oxorhenate Anions". Inorganic Syntheses. pp. 257–262. doi:10.1002/9780470132623.ch42. ISBN 9780470132623.
- Klemperer, Walter G. (1990). "Tetrabutylammonium Isopolyoxometalates". Inorganic Syntheses. Vol. 27. pp. 74–85. doi:10.1002/9780470132586.ch15. ISBN 9780470132586.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.