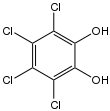
Tetrachlorocatechol
Tetrachlorocatechol is an organochlorine compound with the formula C6Cl4(OH)2. It is a white solid. It results from the degradation of the controversial pesticide pentachlorophenol.[2] It is a precursor to the reagent TRISPHAT. Its conjugate base also functions as a ligand for transition metals.[3]
 | |
| Names | |
|---|---|
| Other names
3,4,5,6-Tetrachloro-1,2-benzenediol, Tetrachloropyrocatechol | |
| Identifiers | |
3D model (JSmol) |
|
| 1876366 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.150.164 |
| EC Number |
|
| 3937 | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H2Cl4O2 | |
| Molar mass | 247.88 g·mol−1 |
| Appearance | white solid |
| Density | 1.848 g/cm3 (20 °C) |
| Melting point | 194 °C (381 °F; 467 K) |
| Hazards | |
| GHS labelling:[1] | |
   | |
| Danger | |
| H302, H318, H400 | |
| P264, P264+P265, P270, P273, P280, P301+P317, P305+P354+P338, P317, P330, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- "Tetrachlorocatechol". pubchem.ncbi.nlm.nih.gov.
- Oturan, Mehmet A.; Oturan, Nihal; Lahitte, Claude; Trevin, Stéphane (2001). "Production of hydroxyl radicals by electrochemically assisted Fenton's reagent". Journal of Electroanalytical Chemistry. 507 (1–2): 96–102. doi:10.1016/S0022-0728(01)00369-2.
- Ackermann, Jens; Meyer, Franc; Kaifer, Elisabeth; Pritzkow, Hans (2002). "Tuning the Activity of Catechol Oxidase Model Complexes by Geometric Changes of the Dicopper Core". Chemistry - A European Journal. 8 (1): 247–258. doi:10.1002/1521-3765(20020104)8:1<247::AID-CHEM247>3.0.CO;2-P. PMID 11822456.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.