Tetrahydroxozincate
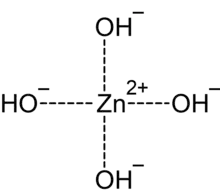
In chemistry, tetrahydroxozincate or tetrahydroxidozincate[1] is a divalent anion (negative ion) with formula Zn(OH)2−
4, with a central zinc atom in the +2 or (II) valence state coordinated to four hydroxide groups. It has Sp3 hybridization. It is the most common of the zincate anions, and is often called just zincate.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| H4O4Zn−2 | |
| Molar mass | 133.41 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
These names are also used for the salts containing that anion, such as sodium zincate Na2Zn(OH)4 [2] and calcium zincate CaZn(OH)4·2H2O [3]
Zincate salts can be obtained by reaction of zinc oxide (ZnO) or zinc hydroxide (Zn(OH)
2) and a strong base like sodium hydroxide.
It is now generally accepted that the resulting solutions contain the tetrahydroxozincate ion.[4] Earlier Raman studies had been interpreted as indicating the existence of linear ZnO2−
2 ions.[5]
Related anions and salts
The name "zincate" may also refer to a polymeric anion with formula approaching [Zn(OH)−
3]n, which forms salts such as NaZn(OH)
3·H
2O,[6] or to mixed oxides of zinc and less electronegative elements, such as Na
2ZnO
2.[7]
See also
- tetrachlorozincate or tetrachloridozincate, ZnCl2−
4 - tetranitratozincate, Zn(NO
3)2−
4
References
- IUPAC nomenclature of inorganic chemistry 2005
- Stahl, R.; Niewa, R.; Jacobs, H. (1999). "Synthese und Kristallstruktur von Na2Zn(OH)4". Zeitschrift für anorganische und allgemeine Chemie. 625: 48–50. doi:10.1002/(SICI)1521-3749(199901)625:1<48::AID-ZAAC48>3.0.CO;2-L.
- Sharma, Ram A. (1986). "Physico-Chemical Properties of Calcium Zincate". Journal of the Electrochemical Society. 133 (11): 2215–2219. Bibcode:1986JElS..133.2215S. doi:10.1149/1.2108376.
- Kaumudi I. Pandya; Andrea E. Russell; J. Mc Breen; W. E. O' Grady (1995). "EXAFS Investigations of Zn(II) in Concentrated Aqueous Hydroxide Solutions". The Journal of Physical Chemistry. 99 (31): 11967. doi:10.1021/j100031a026.
- Sharma, Shiv Kumar (1973). "Raman study of aqueous zinc oxide–alkali metal hydroxide system". The Journal of Chemical Physics. 58 (4): 1626–1629. Bibcode:1973JChPh..58.1626S. doi:10.1063/1.1679405.
- R. Stahl; H. Jacobs (1998). "Synthese und Kristallstruktur von NaZn(OH)3·H2O und NaZn(OH)3". Zeitschrift für anorganische und allgemeine Chemie. 624 (1): 25–29. doi:10.1002/(SICI)1521-3749(199801)624:1<25::AID-ZAAC25>3.0.CO;2-8.
- D. Trinschek; M. Jansen (1996). "Na2ZnO2, ein neues Natriumzinkat". Z. Naturforsch. 51 b: 711–4. doi:10.1515/znb-1996-0515. S2CID 96961116.