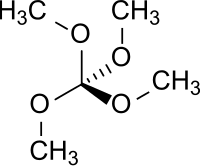
Tetramethoxymethane
Tetramethoxymethane is a chemical compound which is formally formed by complete methylation of the hypothetical orthocarbonic acid C(OH)4 (orthocarboxylic acid violates the Erlenmeyer rule and is unstable in free state).
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Tetramethoxymethane | |
| Other names
Tetramethyl orthocarbonate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.015.853 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 3272 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H12O4 | |
| Molar mass | 136.15 g·mol−1 |
| Appearance | colourless liquid[1] |
| Density | 1.023 g/cm3 (25 °C) |
| Melting point | −5.5 °C[1] |
| Boiling point | 114 °C[1] |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H225, H315, H319, H335 | |
| P210, P261, P305+P351+P338 | |
| Related compounds | |
Other cations |
Tetramethoxysilane |
Related compounds |
Tetraethoxymethane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
The obvious synthetic route from tetrachloromethane does not yield the desired product.[2] The original preparation of the tetramethoxymethane was therefore based on chloropicrin:[1]
![]()
Because of the unpleasant properties of the chloropicrin, other tetrasubstituted reactive methane derivatives were investigated as starting material for tetramethoxymethane. For example, trichloromethanesulfenyl chloride (also used as a chemical warfare agent and easily accessible from carbon bisulfide and chlorine) was used:[3][4]
![]()
A less problematic synthesis is based on trichloroacetonitrile,[5][6] with yields of about 70% be achieved:
![]()
Further preparative methods are described in the literature.[7]
Properties
Tetramethoxymethane is water-clear, aromatic-smelling, low-viscosity liquid which is stable against peroxide formation.[8]
Use
In addition to the use as a solvent, tetramethoxymethane is used as a fuel in polymer fuel cells,[9] as an alkylating agent at elevated temperatures (180-200 °C)[10] as a transesterification reagent (but showing less reactivity than trimethoxymethane[2]) and as a reagent for the synthesis of 2-aminobenzoxazoles, which are used as molecular building blocks in pharmaceutical active ingredients used in neuroleptics, sedatives, antiemetics, muscle relaxants, fungicides and others.[11]
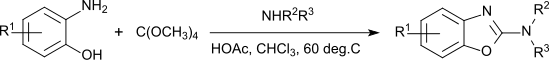
Depending on the substituents, the one pot reaction proceeds in "modest to excellent" yields.
References
- H. v. Hartel, Über Existenz und Darstellung des Orthokohlensäure-tetramethylesters, Ber.dtsch.chem.Ges., 60(8), 1841 (1927), doi:10.1002/cber.19270600821.
- R. H. De Wolfe, Carboxylic ortho acid derivatives: preparation and synthetic applications, Organic Chemistry, Vol. 14, Academic Press, Inc. New York – London, 1970, ISBN 978-0-12-214550-6.
- H. Tieckelmann, H. W. Post, The preparation of methyl, ethyl, propyl, and butyl orthocarbonates, J. Org. Chem., 13 (2), 265-267 (1948), doi:10.1021/jo01160a014.
- US-Patent US 4,059,656, Processes for neutralizing 2,3-dibromopropanol phosphoric acid esters contained in tris(2,3-dibromo-1-propyl) phosphate, Erfinder: M. Demarcq, Anmelder: Produits Chimiques Ugine Kuhlmann, erteilt am 22. November 1974.
- US-Patent US 3,876,708, Orthocarbonic acid esters, Erfinder: R. Speh, W. Kantlehner, Anmelder: Akzo B.V., erteilt am 8. April 1975.
- US-Patent US 6,825,385 B2, Process for the preparation of orthocarbonates, Erfinder: G. Fries, J. Kirchhoff, Anmelder: Degussa AG, erteilt am 30. November 2004.
- W. Kantlehner et al., Die präparative Chemie der O- und N-funktionellen Orthokohlensäure-Derivate, Synthesis; 1977(2): 73-90, doi:10.1055/s-1977-24283.
- K. R. Kopecky; J. Molina (1987). "Bis(dimethoxymethyl) peroxide and bis(1,1-dimethoxyethyl) peroxide". Canadian Journal of Chemistry. 65 (10): 2350. doi:10.1139/v87-392.
- US-Patent US 6,864,001, Tetramethyl orthocarbonate fuel cells and systems and methods related thereto, Erfinder: J. Zhang, K. Colbow, Anmelder: Ballard Power Systems Inc., erteilt am 8. März 2005.
- M. Selva et al., Esters and Orthoesters as Alkylating Agents at High Temperature. Applications to Continuous-flow Processes, J. Chem. Soc., Perkin Trans. 2, 519 (1992), doi:10.1039/P29920000519.
- C. L. Cioffi et al., Synthesis of 2-Aminobenzoxazoles Using Tetramethyl Orthocarbonate or 1,1-Dichloro-diphenoxymethane, J. Org. Chem., 75 (2), 7942-7945 (2010), doi:10.1021/jo1017052.